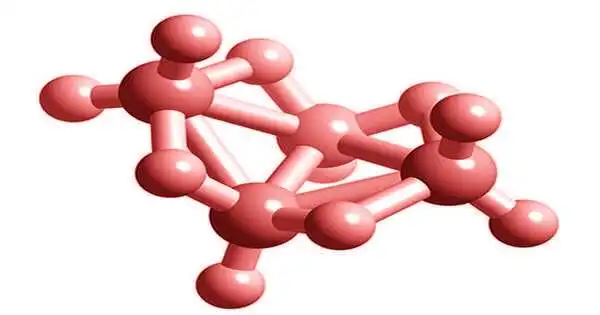
Tetraborane, B4H10, is one of several stable binary boron-hydrogen compounds. It was isolated by Alfred Stock and was the first boron hydride compound to be classified by Stock and Messenez in 1912. It is a gas with the characteristic musty odor of boron hydrides and, like the others, is toxic to the central nervous system. It has a relatively low boiling point of 18 °C and is a gas at room temperature. It forms intriguing coordinate bonds with trifluorophosphine.
Tetraborane gas has a strong odor and is toxic. It can be absorbed through the skin, which increases its toxicity.
Properties
- Chemical formula: B4H10
- Molar mass: 53.32 g/mol
- Appearance: colorless gas
- Density: 2.3 kg m−3 (gas)
- Melting point: −120.8 °C (−185.4 °F; 152.3 K)
- Boiling point: 18 °C (64 °F; 291 K)
History
Lipscomb et al. discovered the class of boranes in the 1950s using X-ray diffraction analysis. The X-ray data revealed multicenter bonds with two electrons. Later, the charge density was analyzed using high-resolution X-ray data.
Preparation
Tetraborane can be created by combining acid with magnesium, aluminum, or beryllium borides. Tetraborane is produced by the hydrolysis of magnesium boride, the high-temperature hydrogenation of boron halides, and the pyrolysis of diborane. One of the first reactions to produce a high yield (14% of tetraborane) was the hydrolysis of magnesium boride. In the reaction with magnesium boride, phosphoric acid proved to be the most efficient acid (other than hydrochloric and sulfuric acid).
Safety
Because it is easily oxidized, it must be kept under vacuum. Tetraborane ignites when it comes into contact with air, oxygen, or nitric acid. Boranes in general, including tetraborane, have been identified as highly toxic and biologically destructive. A study involving small daily doses of the chemical administered to rabbits and rats resulted in death.