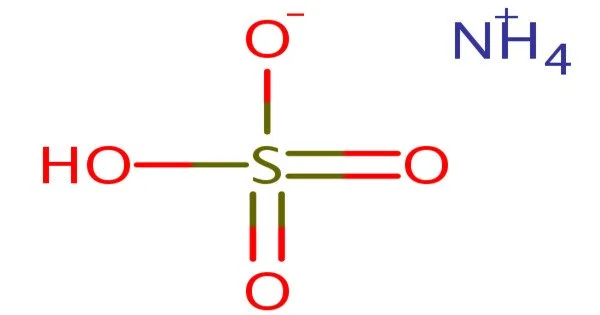
Ammonium bisulfate (NH4)HSO4 is a white, crystalline solid with the formula (NH4)HSO4. It is a sulfuric acid ammonium salt that is used to study chemical reactions involving ammonium salt sulfur acids. This salt is the result of ammonia’s half-neutralization of sulfuric acid.
It is used as an oxygen scavenger in wastewater and pipes to remove dissolved oxygen. Ammonium bisulfite is a bleaching agent that is reductive in nature. It reduces the carbonyl and alcohol groups, which serve as the substances’ colorants. It’s used to bleach mechanical paper pulp, cotton, wool, and kaolin clay, among other things. Other uses include being a preservative (antioxidant) and a hair-waving agent.
Properties
Ammonium hydrogen sulfate is a colorless to white, powdered solid. It is toxic by ingestion. When heated to high temperatures, it may release toxic sulfur oxide and nitrogen oxide fumes. It is soluble in water. It is a chemical catalyst, used in hair preparations.
- Chemical formula: (NH4)HSO4
- Molar mass: 115.11 g/mol
- Appearance: White solid
- Density: 1.78 g/cm3
- Melting point: 147 °C (297 °F; 420 K)
- Solubility in water: Very soluble
- Solubility in other solvents: Soluble in methanol; insoluble in acetone

Production
It is commonly collected as a byproduct of the “acetone cyanohydrin route” to the commodity chemical methyl methacrylate.
It can also be obtained by hydrolysis of sulfamic acid in aqueous solution, which produces the salt in high purity:
H3NSO3 + H2O → [NH4]+[HSO4]–
It also arises by the thermal decomposition of ammonium sulfate:
(NH4)2SO4 → (NH4)HSO4 + NH3
Ammonium bisulfite is a bleaching agent that is reductive. It reduces carbonyl and alcohol groups, which serve as the substances’ colorants. It can be found in the bleaching of mechanical paper pulp, cotton, wool, and kaolin clay.
Other uses include a preservative (antioxidant) and a hair waving agent. Ammonium bisulfite is used in a variety of industries, including leather processing, food and beverage processing, gas purification, water treatment to remove excess chlorine, textile and pulp processing, and many more. It is used as a reducing agent in the chemical industry. It is an alternative to sulfur dioxide, which is used in equipment sterilization.
Applications
Ammonium bisulfite is used in a variety of industries, including leather processing, food and beverage processing, gas purification, water treatment to remove excess chlorine, textile and pulp processing, and many more. It is used as a reducing agent in the chemical industry. It is an alternative to sulfur dioxide, which is used in equipment sterilization.
It can be neutralized further with ammonia to produce ammonium sulfate, a valuable fertilizer. Although sodium bisulfate is far more common, it can be used as a weaker substitute for sulfuric acid.
Natural occurrence
A related compound of the (NH4)3H(SO4)2 formula, occurs as the rare mineral letovicite, known from coal fire environments.