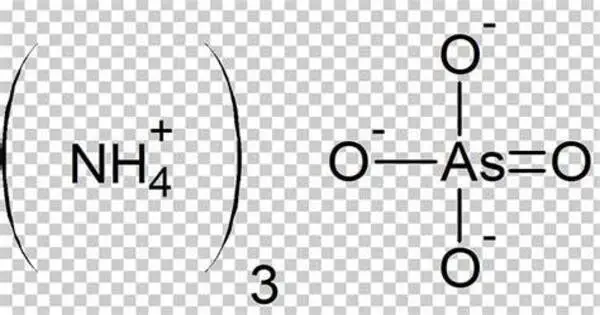Ammonium arsenate is an inorganic compound with the formula (NH4)3AsO4. It is a white, crystalline solid that is highly soluble in water. It is prepared by treating a concentrated solution of arsenic acid with ammonia, resulting in the precipitation of colorless crystals of the trihydrate. Upon heating, it releases ammonia. It is primarily used as a pesticide and herbicide. However, due to its toxic nature, it has largely been phased out in favor of safer alternatives.
Like other compounds of arsenic, it is classified as an IARC Group 1 carcinogen, i.e. carcinogenic to humans. Acid salts are also known, including diammonium arsenate and ammonium dihydrogen arsenate. It may react with acids, bases, and other chemicals to produce toxic or hazardous products.
Properties
- Chemical formula: (NH4)3AsO4.3 H2O
- Molar mass: 247.1 (trihydrate)
- Solubility in water: Soluble
- Molecular Weight: Approximately 283.01 g/mol
- Physical State: It is typically found as a white crystalline solid.
- Solubility: It is highly soluble in water, meaning it readily dissolves in it.
- pH: In aqueous solution, it would be slightly acidic due to the presence of arsenic acid.
Uses: Historically, ammonium arsenate has been used as an herbicide and insecticide. However, its use has declined due to environmental and health concerns associated with arsenic compounds.
Uses
Historically, ammonium arsenate has been used as an herbicide and insecticide. However, its use has declined due to environmental and health concerns associated with arsenic compounds. It should be stored in a cool, dry place away from incompatible substances.
Toxicity
Arsenic compounds like ammonium arsenate are highly toxic and can cause serious health problems if ingested, inhaled, or absorbed through the skin. Chronic exposure to arsenic compounds has been linked to various health issues including skin lesions, cancer, cardiovascular diseases, and neurological disorders.
Safety Precautions
When handling ammonium arsenate, appropriate safety measures should be taken, including the use of gloves, goggles, and a lab coat to prevent skin contact or inhalation of the compound.