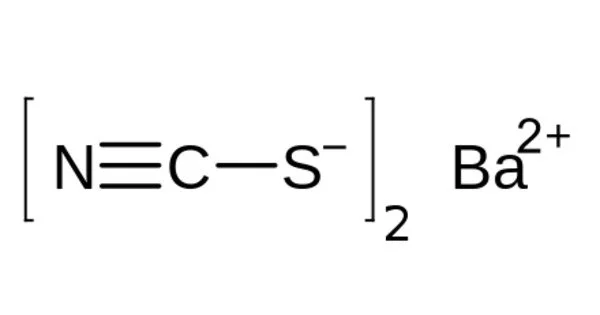Barium thiocyanate is a colorless, water-soluble, and highly hygroscopic salt. It is extremely toxic when consumed and irritates the skin. In addition, it is soluble in most alcohols but insoluble in simple alkanes. It is used in dyeing, photography, the preparation of thiocyanates of other metals, and as a cellulose dispersing agent.
Many reactions generate heat; in fact, heat generation is often expected when people think of chemical reactions. Endothermic reactions, or those that consume heat, can be just as exciting. One of the most striking examples is the mixture of the solids barium hydroxide and ammonium thiocyanate in a beaker.
Properties
- Chemical formula: Ba(SCN)2
- Molar mass: 253.49 g/mol
- Appearance: White crystals
- Solubility in water: 62.63 g/100 ml (25°C)
- Solubility: Soluble in acetone, methanol, and ethanol

Preparation
Barium thiocyanate is made by dissolving barium metal or barium nitrate in thiocyanic acid.
Ammonium thiocyanate is combined with barium hydroxide. The mixture is stirred, and the two solids react. Because water is one of the reactions products, slush is formed. A drop of water is placed on a block of wood, and the beaker is placed on top of the water drop. The reaction is endothermic, meaning it absorbs heat. As a result, the bottom of the beaker becomes cold enough to freeze the water and cause it to stick to the wood.
Uses
Barium thiocyanate is used in textile dyeing and is found in some photographic solutions. However, due to its toxicity, it has limited applications.
Ingestion of barium salts is toxic. Ingestion of ammonium thiocyanate is also hazardous. Handle the beaker or flask with care. If tongs are available, use them. The temperatures involved are cold enough to cause skin to freeze. Ammonia vapor is extremely irritating to the eyes and respiratory tract. Students should not be allowed to inhale this gas. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron when working with chemicals.