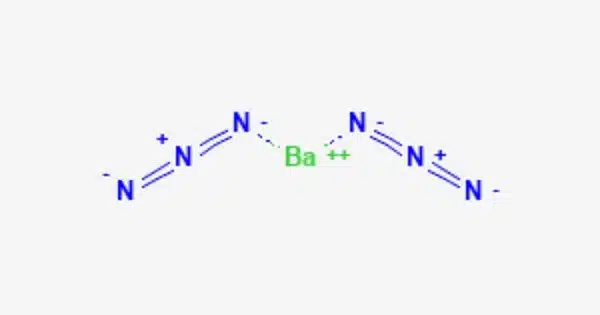
Barium azide, with the formula Ba(N3)2, is an inorganic azide. It is a hydrazoic acid barium salt. It is explosive, as are most azides. It’s an azide compound made up of barium and the azide ion (N3-). Azides, including barium azide, are notorious for their explosive qualities. It has a lower mechanical shock sensitivity than lead azide.
It is extremely sensitive and can explode when subjected to mechanical force or friction. Because of its explosive nature, barium azide is largely employed in the production of initiators and detonators for use in pyrotechnics and explosives.
Preparation
Barium azide can be synthesized by reacting sodium azide with a soluble barium salt. Large crystals should be avoided in the solution since barium azide crystals will explode if subjected to friction/shock or completely dried. The product should be stored submerged in ethanol.
Properties
- Chemical formula: Ba(N3)2
- Molar mass: 221.37 g/mol
- Appearance: White crystalline solid
- Odor: Odourless
- Density: 2.936 g/cm3
- Melting point: 126 °C (259 °F; 399 K)
- Boiling point: 160 °C (320 °F; 433 K) (initial decomposition) >217 °C (deflagrates)
- 180 °C (initial decomposition), 225 °C explosion
- Solubility in water: 11.5 g/100 mL (0 °C); 24.75 g/100 mL (70 °C)
- Solubility in ethanol: 0.017 g/100 mL (16 °C)
- Solubility in acetone: Insoluble
- Solubility in ether: Insoluble
- Crystal structure: Monoclinic
Uses
Barium azide can be used to make azides of magnesium, sodium, potassium, lithium, rubidium and zinc with their respective sulfates.
Ba(N3)2 + Li2SO4 → 2 LiN3 + BaSO4
It can also be used as a source for high purity nitrogen by heating:
Ba(N3)2 → Ba + 3 N2
This reaction liberates metallic barium, which is used as a getter in vacuum applications.
Safety
Barium azide should be handled with considerable attention due to its tremendous sensitivity and potential risks, and it is normally utilized by skilled professionals who take the essential safety procedures. When working with such chemicals, it is critical to adhere to all safety norms and regulations in order to avoid accidents and protect the safety of both humans and the environment.