An oxidizing acid is a strong oxidizing agent that is a Brnsted acid. These are chemical compounds with both acidic and oxidizing properties. Because acidic proton can be reduced to hydrogen gas, most Brnsted acids can act as oxidizing agents. They can donate protons (H+) while also facilitating oxidation reactions by accepting electrons from other substances. Other structures in some acids act as stronger oxidizing agents than hydrogen ions. These acids have the ability to react with a wide range of materials, including metals, nonmetals, and organic compounds. They usually have oxygen in their anionic structure. Among these are nitric acid, perchloric acid, chloric acid, chromic acid, and concentrated sulfuric acid.
Nitric acid (HNO3) is an example of an oxidizing acid. It is a strong and highly corrosive acid that is commonly used in laboratories and industrial settings. While undergoing reduction, nitric acid can donate a proton, resulting in the formation of nitrate ions (NO3-). It is also a powerful oxidizing agent, capable of oxidizing metals like copper to higher oxidation states or nonmetals like sulfur to higher oxidation states.
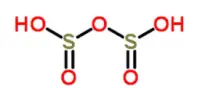
Another case in point is sulfuric acid (H2SO4). It is a strong acid with a high oxidizing capacity. Sulfuric acid has the ability to donate two protons while also acting as an oxidizing agent by accepting electrons from other substances. It is widely used in a variety of industrial processes, such as the manufacture of fertilizers, dyes, and detergents.
General properties
Because oxidizing acids are strong oxidizing agents, they can frequently oxidize certain less reactive metals where the active oxidizing agent is not H+ ions. Copper, for example, is a relatively unreactive metal that has no reaction with concentrated hydrochloric acid. Even dilute nitric acid, on the other hand, can oxidize copper to Cu2+ ions, with nitrate ions acting as the effective oxidant:
3 Cu + 8 HNO3 → 3 Cu2+ + 2 NO + 4 H2O + 6 NO−3
The concentration of the acid can sometimes be a factor in its ability to be strongly oxidizing. Copper has no reaction with dilute sulfuric acid, but the highly acidic environment and high concentration of sulfate ions in concentrated sulfuric acid allow the sulfate ions to act as an oxidizing agent. Although sulfuric acid is not an oxidizing agent, the sulfate ion is a weak oxidizing agent. Sulfur cannot act as a reducing agent because it is in its maximum oxidation state as the sulfate ion.
Cu + 2 H2SO4 → SO2 + 2 H2O + SO2−4 + Cu2+
Perchloric acid (HClO4), chromic acid (H2CrO4), and periodic acid (HIO4) are examples of oxidizing acids. Each of these acids has unique properties and uses in a variety of chemical reactions and industries.
Applications
Oxidizing acids have various applications in industries and laboratories. They are used in the production of fertilizers, dyes, explosives, and pharmaceuticals. They are also utilized in etching and cleaning processes, as well as in the synthesis of organic compounds.
It’s important to note that oxidizing acids can be highly reactive and potentially hazardous. Proper safety precautions should be taken when handling and using these substances to prevent accidents or injuries.