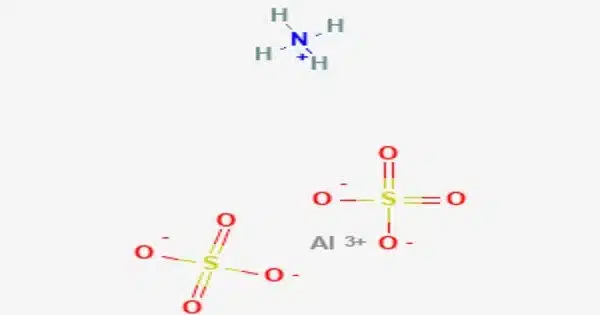
Ammonium aluminium sulfate, also known as ammonium alum or simply alum, is a white crystalline double sulfate that is commonly found as a dodecahydrate, formula (NH4)Al(SO4)2•12H2O. It is used sparingly in a variety of niche applications. Tschermigite is a rare mineral that contains dodecahydrate.
Ammonium alum has several uses, including as a mordant in dyeing and printing textiles, as a food additive to preserve and firm pickles, and as an ingredient in baking powder. It is also used in water purification, papermaking, and as a flame retardant.
Properties
Ammonium Aluminium Sulfate is a colorless or white crystalline solid that is soluble in water. It is a double salt, consisting of ammonium sulfate and aluminum sulfate. It is used in a variety of applications, including water purification, as a mordant in dyeing fabrics, and in the manufacture of baking powder and other aluminum compounds.
- Chemical formula: (NH4)Al(SO4)2
- Molar mass: 237.15 g/mol (anhydrous); 453.33 g/mol (dodecahydrate)
- Appearance: white crystals
- Density: 2.45 g/cm3 (anhydrous); 1.64 g/cm3 (dodecahydrate)
- Melting point: 93.5 °C (200.3 °F; 366.6 K) (dodecahydrate)
- Boiling point: 120 °C (248 °F; 393 K) dehydr. (dodecahydrate)
- Solubility in water: 15 g/100 ml (20 °C, dodecahydrate)
- Crystal structure: Hexagonal (anhydrous)
- Coordination geometry: Octahedral (Al3+)
Production
Aluminium hydroxide, sulfuric acid, and ammonium sulfate are used to make ammonium alum. It creates a solid solution with potassium alum. Pyrolysis generates alumina. Such alumina is used to make grinding powders and as a precursor to synthetic gems.
Uses
Ammonium alum is not a major industrial chemical or a particularly useful laboratory reagent, but it is inexpensive and effective, which makes it suitable for a wide range of niche applications. It is used in water purification, vegetable glues, porcelain cement, deodorants, textile tanning, dyeing, and fireproofing. The pH of the solution produced by topical application of ammonium alum with perspiration is typically in the slightly acidic range, ranging from 3 to 5.
In addition, ammonium alum has medicinal properties and has been used historically as an astringent and styptic to stop bleeding and treat wounds. However, its use in medicine is limited today due to the availability of more effective and safer alternatives.
Toxicity
Ammonium Aluminium Sulfate is generally considered safe for use in food and pharmaceuticals when used in accordance with appropriate regulations. However, ingestion of large quantities may cause gastrointestinal distress.