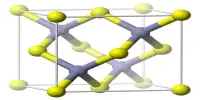
Aluminium selenide (Al2Se3) is a compound made up of two aluminium atoms and three selenium atoms. It is a dark grey or black powder that is insoluble in water and has a melting point of 1300°C.
Aluminium selenide is a semiconductor material and has potential applications in electronic devices such as solar cells, sensors, and photodetectors. It has also been studied for its potential use in thin film coatings and as a catalyst in chemical reactions.
Properties
Aluminum selenide is a gray crystalline solid with a melting point of 1150°C and a density of 4.61 g/cm3. It is insoluble in water but soluble in acids. It is a semiconductor material with a bandgap of around 2.2 eV. It has been used in the development of optoelectronic devices such as solar cells, light-emitting diodes (LEDs), and photodetectors.
- Chemical formula: Al2Se3
- Molar mass: 290.84 g/mol
- Appearance: yellow to brown powder
- Odor: odorless
- Density: 3.437 g/cm3
- Melting point: 947 °C (1,737 °F; 1,220 K)
- Solubility in water: decomposes
- Crystal structure: Monoclinic
Preparation
It is a solid prepared by igniting a mixture of the elements at 1,000 °C (1,830 °F):
2 Al + 3 Se → Al2Se3
The pure compound is white, but typical samples are coloured. Samples of aluminium selenide must be protected from moisture, because the compound hydrolyzes readily, giving off highly toxic hydrogen selenide gas:
Al2Se3 + 3 H2O → Al2O3 + 3 H2Se
Uses
Al2Se3 has been used as a precursor to hydrogen selenide, which is released when the solid is treated with acids.
However, it is important to note that aluminium selenide is a toxic substance and should be handled with care. Exposure to the compound can lead to respiratory and skin irritation, and ingestion or inhalation can be harmful to human health. Therefore, appropriate safety measures and protective equipment should be used when working with aluminium selenide.
Safety
Aluminium selenide should be stored and handled away from moisture and air. It is a toxic material and should be handled with care. It can cause skin and eye irritation, as well as respiratory problems if inhaled.