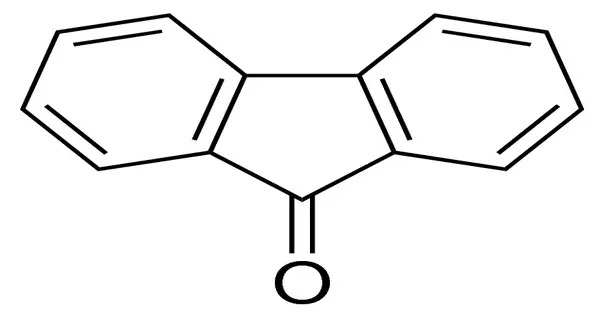
Fluorenone is an organic compound with the chemical formula (C6H4)2CO. It is bright fluorescent yellow solid. It consists of a fluorene backbone with a ketone group (C=O) attached at the 9-position, making it a ketone derivative of fluorene.
Fluorenone is relatively stable under normal conditions, although it can undergo typical reactions associated with carbonyl compounds such as reduction, oxidation, and nucleophilic addition. It derivatives have applications in organic synthesis, particularly as intermediates in the production of various organic compounds. It is also used in some research applications.
Properties
- Physical State: At room temperature, fluorenone appears as a yellow crystalline solid.
- Melting Point: The melting point of fluorenone is around 84-85°C.
- Reactivity: The carbonyl group in fluorenone makes it susceptible to nucleophilic addition reactions and other reactions typical of carbonyl compounds.
Synthesis and reactions
It is synthesised by aerobic oxidation of fluorene:
(C6H4)2CH2 + O2 → (C6H4)2CO + H2O
Fluorenone sustains up to four nitro groups giving 2,4,5,7-tetranitrofluorenone. It is sparingly soluble in water but more soluble in organic solvents such as acetone, ether, benzene, and chloroform.
Applications
Fluorenone is often used as a precursor in organic synthesis reactions to introduce the fluorenone moiety into more complex molecules. Its unique structure and reactivity make it valuable in the preparation of various organic compounds. Several substituted fluorenones are biologically active as antibiotic, anticancer, antiviral, or neuromodulatory compounds.
Some substituted azafluorenones are biologically active, such as the naturally occurring antimicrobial compound onychine (1-methyl-4-azafluorenone). The compound 1,8-diazafluoren-9-one is used for fingerprint detection.
Toxicity
Fluorenone is not considered highly toxic, but like many chemicals, it should be handled with care, and exposure should be minimized.