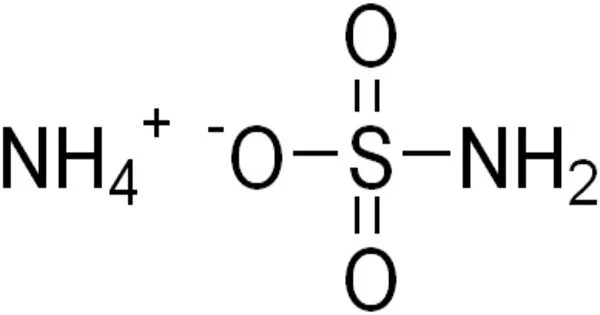Ammonium sulfamate is a white crystalline substance that dissolves easily in water. It is a molecular organic entity. It is most typically employed as a broad-spectrum herbicide, although it can also be used as a compost accelerator, a flame retardant, and in industrial operations. It’s a salt made of ammonia and sulfamic acid. It is a herbicide that is used to control a wide range of woody plants, trees, herbaceous perennials, as well as annual broadleaf weeds and grasses. It is also utilized in the printing and dyeing industries, as well as the tobacco, building material, and textile industries.
The main risk is the harm to the environment. Immediate action should be done to limit its environmental spread. It is used to flameproof fabrics and papers, as well as in weed and brush-killing goods and other applications.
Properties
- Chemical formula: [NH4]SO3NH2
- Molar mass: 114.125 g/mol
- Appearance: White solid hygroscopic
- Density: 1.8 g/cm3
- Melting point: 131 °C (268 °F; 404 K)
- Boiling point: 160 °C (320 °F; 433 K) (decomposes)
- Solubility in water: very soluble
- Solubility: soluble in glycerol, glycol, formamide; slightly soluble in ethanol

Manufacture and distribution
It is a salt formed from ammonia and sulfamic acid.
Ammonium sulfamate is distributed under the following tradenames, which are principally herbicidal product names: Amicide, Amidosulfate, Ammate, Amcide, Ammate X-NI, AMS, Fyran 206k, Ikurin, Sulfamate, AMS and Root-Out.
Uses
- Herbicide
Ammonium sulfamate is thought to be very effective at controlling strong woody plants, tree stumps, and brambles. It has been utilized effectively in a number of large UK projects by organizations such as the British Trust for Conservation Volunteers, English Heritage, the National Trust, and numerous railway, canal, and waterway authorities.
After a successful series of herbicide trials, the Henry Doubleday Research Association (HDRA) (also known as Garden Organic) published an essay on ammonium sulfamate few years ago. Although it is not permitted for use by organic gardeners, it does provide an alternative when other options have failed.
- Compost accelerator
Ammonium sulfamate is used as a composting accelerator in horticultural settings. It is especially effective in breaking down the tougher and woodier weeds put onto the compost heap.
- Flame retardant
Ammonium sulfamate (together with other ammonium compounds such as ammonium dihydrogen phosphate and ammonium sulfate) is an effective flame retardant. These salt-based flame retardants are water processable, which gives them an advantage over other metal/mineral-based flame retardants. Because of their low decomposition temperature, they are ideal for flame retarding cellulose-based materials (paper/wood). Ammonium sulfamate (like ammonium dihydrogen phosphate) is sometimes used with Magnesium sulfate or Ammonium sulfate (in around 2:1 ratios) to improve flame retardant characteristics.
- Other uses
Within industry ammonium sulfamate is used as a flame retardant, a plasticiser and in electro-plating. Within the laboratory it is used as a reagent.
Safety
Ammonium sulfamate is only mildly hazardous to people and other animals, making it suitable for amateur home gardening, professional and forestry applications. It is generally regarded as safe for use on areas of land dedicated to growing fruit and vegetables for human consumption. It is also thought to be environmentally favorable due to the fact that it degrades to non-harmful residues.