Carbonaceous sulfur hydride is a group of compounds containing carbon, sulfur, and hydrogen atoms. The presence of covalent bonds between carbon and sulfur atoms, as well as carbon and hydrogen atoms, distinguishes these compounds. Carbonaceous sulfur hydrides can have a wide range of chemical and physical properties depending on their molecular structure and atom arrangement.
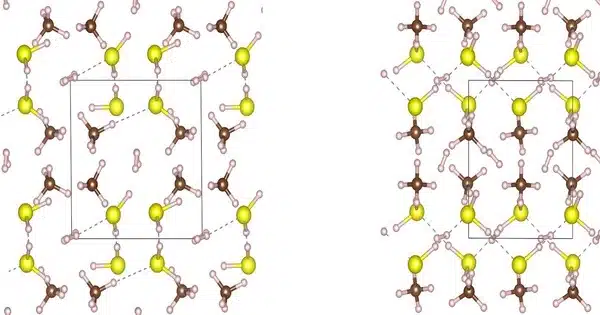
Carbonaceous sulfur hydride, which was announced in October 2020, is a purported room-temperature superconductor. The material is claimed to have a maximum superconducting transition temperature of 15 degrees Celsius (59 degrees Fahrenheit) at a pressure of 267 gigapascals (GPa), though the validity of the claim has been questioned. The article was retracted in September 2022 by the Nature Journal editorial board due to a non-standard, user-defined data analysis that called the scientific validity of the claim into question.
267 GPa is the pressure equivalent to three-quarters of the pressure at the Earth’s center. The substance is an unidentified ternary polyhydride compound of carbon, sulfur, and hydrogen with the chemical formula CH8S.
Properties
Carbonaceous sulfur hydrides can have a variety of chemical and physical properties depending on how the atoms are arranged and substituted. They have a wide range of applications in organic synthesis, industrial processes, and even biological systems. Carbonaceous sulfur hydrides are still being researched and developed for their potential applications and properties.
Extreme pressure measurements are difficult, and the elements, in particular, are too light for X-ray crystallography to determine crystal structure. With an onset nearly 30 °C higher than the previous record-holder, this would be the closest to room temperature achieved for a superconductor.
Thiophene (C4H4S), a five-membered ring with four carbon atoms and one sulfur atom, is an example of a carbonaceous sulfur hydride. Thiophene is commonly found in fossil fuels such as crude oil and coal and is widely used as a building block in the synthesis of various organic compounds. It is well-known for its potent and distinct odor.
Hydrogen sulfide (H2S) is another well-known carbonaceous sulfur hydride, consisting of two hydrogen atoms bonded to a sulfur atom. Hydrogen sulfide is a colorless gas with a distinct rotten egg odor. It is naturally produced by biological processes such as organic matter decomposition and the activity of sulfur-reducing bacteria. Hydrogen sulfide is also used in various industrial applications, including the production of sulfuric acid and as a reducing agent.