Conductivity Water
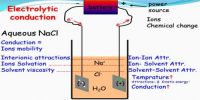
For conductance measurements it is necessary to use very pure water because the impurities, if present, might contribute to the total conductance, thereby giving incorrect results. Water for conductance measurements, known as conductivity water, can be obtained by three distillations of ordinary water with addition of small amounts of potassium permanganate in an all glass (pyrex) distillation set. Purer water can be obtained by using tin-plated copper condensers.
Alternatively, the water may be passed through mixed bed ion-exchange resins when de-ionized water can be obtained. For most purposes the water should not have a specific conductance more than 2 x 10-6 0ohm-1 cm-1 at 25°C.
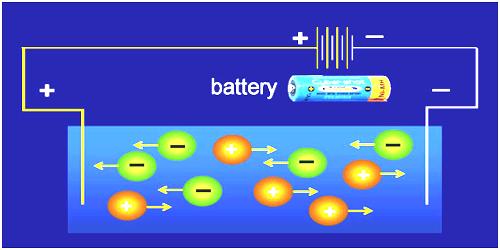
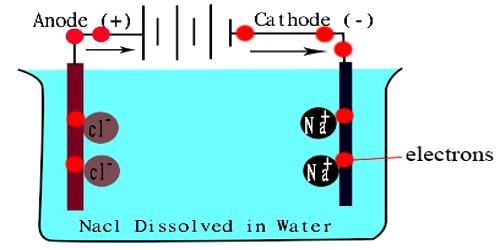
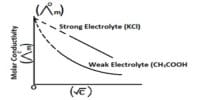
Pure water is not a good conductor of electricity. Ordinary distilled water in equilibrium with carbon dioxide of the air has a conductivity of about 10 x 10-6 W-1 m-1 (20 dS/m). Because the electrical current is transported by the ions in solution, the conductivity increases as the concentration of ions increases.
Thus conductivity increases as water dissolved ionic species.