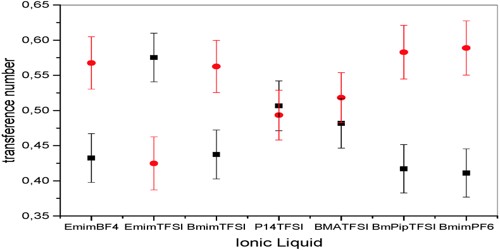
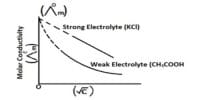
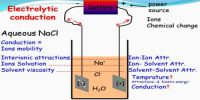
Transference number of an ion is the fraction of the total current that is carried by that ion during electrolysis. In an electrolytic solution the current is carried by both cations and anions. In a solution of an electrolyte where the number of ions is fixed or does not vary much, the conductance would be mainly governed by ionic velocities. Different ions carry different fractions of the current because different ions move at different speeds under the same potential gradient. If u+ represents the ionic velocity of the cation and u– that of the anion then the current carried by the cation will be proportional to u+ and the current carried by anion will be proportional to u–. The total current carried through the solution will be proportional to (u+ + u–).
The fraction of the current curried by each ionic species is called the transport number of that ion.
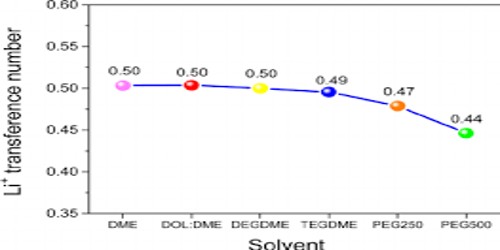
The transport number is generally determined by the Hittorf method—that is, by the transform in the concentrations of the ions near the electrodes. During electrolysis, ions, owing to solvation, transport not only electric charge but also the solvent in their solvation sheaths. For this cause, transport numbers determined by the Hittorf method are called apparent transport numbers—in contrast to true transport numbers, which take into account only the velocities of the ions.