Aluminum(I) oxide, also known as aluminum monoxide, is a chemical compound with the formula Al2O. It can be prepared by heating the stable oxide Al2O3 with elemental silicon at 1800 °C under vacuum. However, this compound is unstable and tends to quickly decompose to aluminum metal and aluminum oxide (Al2O3), which is the stable form of aluminum oxide.
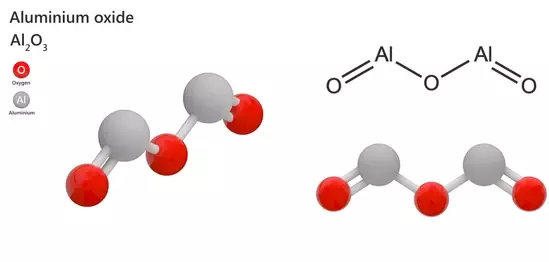
Aluminum oxide (Al2O3) is a white or nearly colorless crystalline powder that is widely used in a variety of applications. It is commonly referred to as alumina and is one of the most widely used materials in engineering ceramics due to its excellent mechanical, thermal, and electrical properties. Aluminum(I) oxide has a high refractive index and is used as a coating material in optical applications.
Properties
Aluminum(I) oxide is a white solid with a hexagonal crystal structure. It is a reactive compound and readily reacts with water to produce aluminum hydroxide. It is also capable of reacting with acids and bases to form salts. It is also a good thermal conductor. It is an insulator and has a high dielectric constant, making it useful in electronic applications.
- Chemical formula: Al2O
- Molar mass: 69.962 g·mol−1
- Melting point: 2,300°C
- Boiling point: 2,977°C
Formation and occurrence
Because the solid state of Al2O is not stable at room temperature and is only stable between 1050 and 1600 °C, it is commonly found as a gas. Aluminium(I) oxide is produced solely by condensing the products of heating Al and Al2O3 in a vacuum while in the presence of SiO2 and C.
This compound is not widely known; it is unstable, has complex high-temperature spectra, and is difficult to detect and identify. In reduction, Al2O is a major component of Al2O3 vapors. Al2O also contains 12 valence electrons. In the gas phase, Al2O molecules can be detected using mass spectrometry, infrared emission, and ultraviolet absorption and emission.
Uses
Aluminium as a metal fuel with oxidizers creates highly exothermic reactions. When Al2O3 is added to a pressure system, the reaction goes from steady, to accelerating, to unstable. This reaction indicates that unstable intermediates such as AlO or Al2O condense or do not form, which prevent acceleration and convection down the pressure system.
Aluminium oxides are used as catalysts and are products of aluminium combustion. It is also used as an abrasive, as a refractory material, and in the production of aluminum metal. It is also used in the production of high-purity alumina, which is used as a substrate for the production of semiconductors and other electronic components. In summary, while Aluminum(I) oxide exists, it is highly unstable and quickly decomposes to Aluminum oxide (Al2O3), which is a widely used material with many important applications.