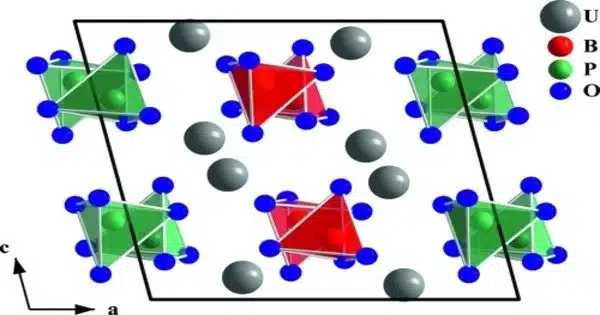
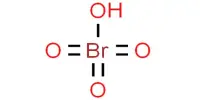
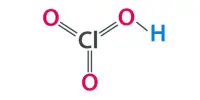
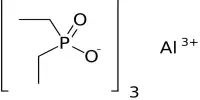
Borate phosphates are a group of chemical compounds that contain both borate and phosphate ions. These compounds can form through the reaction of borate and phosphate minerals or through the thermal decomposition of borate and phosphate salts. Borate phosphates can have a wide range of properties depending on their composition and structure, but they generally have good thermal stability and are resistant to chemical corrosion.
Borate phosphates are anion compounds that contain both borate and phosphate anions. They differ from borophosphates in that the borate is linked to the phosphate via a common oxygen atom. When compared to borophosphates, borate phosphates have a higher ratio of cations to borates and phosphates.
NADH-borate is an example of an organic ester of both borate and phosphate.
Properties
- Borate phosphates can have a wide range of properties depending on their chemical composition and the conditions under which they are synthesized. Some general properties of borate phosphates include:
- Solubility: These are typically soluble in water and can form clear or slightly turbid solutions.
- Thermal stability: These are generally thermally stable and can withstand high temperatures without decomposing.
- Acid resistance: These are often resistant to acids and can be used in acidic environments without significant degradation.
- Structural complexity: These can have complex structures with multiple borate and phosphate groups arranged in different ways.
- Optical properties: Some borate phosphates exhibit interesting optical properties such as fluorescence, which makes them useful in certain applications.
Production
In the high temperature method, ingredients are heated together at atmospheric pressure. Products are anhydrous, and production or borophosphates is likely.
The boron flux method involves dissolving ingredients such as an ammonium phosphate and metal carbonate in an excess of molten boric acid.
Use
Borate phosphates are of research interest for their optical, electrooptical or magnetic properties.
Some common examples of borate phosphates include boracite (Mg3B7O13Cl), colemanite (Ca2B6O11·5H2O), and clinobisvanite (Na2Bi(PO4)(BO3)). These compounds have a variety of uses, including as flame retardants, ceramic materials, and fertilizers. In addition, some borate phosphates have been studied for their potential applications in optical and electronic devices due to their unique optical and electrical properties.