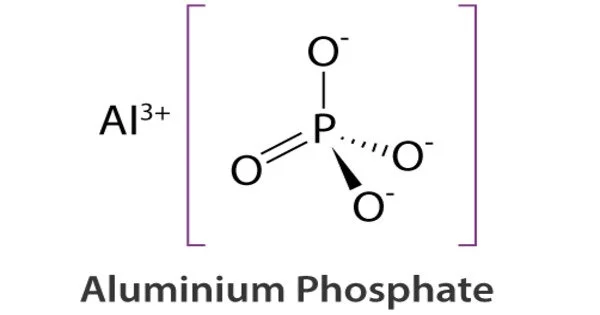
Aluminium phosphate is a chemical compound with the formula AlPO4. It occurs naturally as the mineral berlinite. It is a white, crystalline powder that is commonly used as a catalyst in the production of chemicals such as gasoline and plastics. There are numerous synthetic forms of aluminum phosphate. They have zeolitic framework structures and are used as catalysts, ion exchangers, and molecular sieves. There is commercially available aluminum phosphate gel. It is also used in the production of ceramics, glass, and fertilizers.
Properties
Aluminum phosphate is a white or colorless crystalline powder with a molecular weight of 122.95 g/mol. It has a density of 2.56 g/cm3 and a melting point of 1,600 °C. It is an acidic salt, and it reacts with bases to form aluminum hydroxide and phosphoric acid. It is also an important industrial catalyst in organic reactions such as dehydration and isomerization.
- Chemical formula: AlPO4
- Molar mass: 121.9529 g/mol
- Appearance: White, crystalline powder
- Density: 2.566 g/cm3, solid
- Melting point: 1,800 °C (3,270 °F; 2,070 K)
- Boiling point: Decomposes
- Solubility in water: 1.89×10−9 g/100 ml
- Solubility product (Ksp): 9.84×10−21
- Solubility: Very slightly soluble in HCl and HNO3
Aluminium phosphate is insoluble in water and organic solvents, but it can be dissolved in acids or alkalis. It has a high melting point of 1,770 °C and is stable at high temperatures. It is also a good thermal and electrical insulator.
Aluminum phosphate is slightly soluble in water and forms acidic solutions. Its solubility increases with increasing pH. It is thermally stable and can withstand high temperatures without decomposing.
Application
Aluminum phosphate is used in a variety of industrial applications including as a catalyst, flame retardant, and as an additive in ceramics, glass, and cement. It is also used in the production of animal feed and as a food additive.
In medicine, aluminium phosphate is used as an adjuvant in vaccines to stimulate the immune system and enhance the effectiveness of the vaccine. However, there has been some concern about the potential toxicity of aluminium in vaccines, and research is ongoing to better understand its safety.
luminum phosphate is generally considered safe and is used in a variety of applications including food additives and pharmaceuticals. However, it can be toxic if ingested in large amounts. Overall, aluminium phosphate is a versatile compound with many useful applications in various industries.