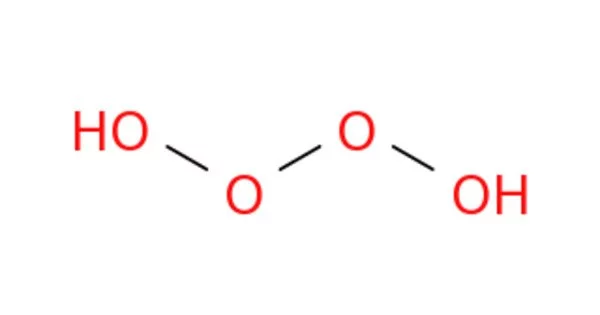
Tetraoxidane is an inorganic hydrogen and oxygen compound with the chemical formula H2O4. This is an example of an unstable hydrogen polyoxide. It is made up of three oxygen atoms linked together in a V-shaped or bent configuration. Ozone is an oxygen allotrope, which means it is a distinct structural form of the same element.
It’s a pale blue gas with a strong, pungent odor. It is most typically found in the stratosphere, where it contributes to the formation of the ozone layer, a region of the atmosphere that absorbs and protects against the majority of the sun’s harmful ultraviolet (UV) radiation. The stratospheric protective role of ozone is critical for protecting life on Earth from the detrimental effects of excessive UV radiation.
It can also be formed near the Earth’s surface as a result of numerous chemical processes, which are frequently caused by human activity. Ground-level ozone, also known as tropospheric ozone, is a primary component of smog and an air contaminant. It can be harmful to human health, causing respiratory problems and other disorders.
Synthesis
The compound is prepared by a chemical reaction between hydroperoxyl radicals (HO2•) at low temperatures:
2HO2∙ → 2H2O4
Properties
- Chemical formula: H2O4
- Molar mass: 66.012 g·mol−1
- Density: 1.8±0.1 g/cm3
Physical properties
This is the polyoxidanes’ fourth member. Water [(mon)oxidane, hydrogen peroxide (dioxidane), and trioxidane are the first three. Tetroxidane is a more unstable chemical than the preceding ones. Beyond the parent chemical, the word “tetraoxidane” refers to a group of daughter compounds with the generic formula R2O4, where R can be hydrogen, halogen atoms, or different inorganic and organic monovalent radicals. Because the two Rs can be substituted by a divalent radical, heterocyclic tetroxidanes are also possible.
Application
Because of its capacity to react quickly with other compounds, ozone is frequently utilized as a potent oxidizing agent in industrial and laboratory settings. It is used to purify water, sterilize it, and remove odors and impurities from air and water.