Cadmium iodide is an inorganic compound with the formula CdI2. It is a cadmium-iodine chemical compound. It is a white hygroscopic solid. It can also be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical of compounds of the form MX2 with strong polarization effects. It is used in lithography, photography, electroplating, and the production
Properties
Cadmium Iodide is a chemical compound made up of the elements cadmium and iodine. It is a green-yellow solid that is soluble in water, alcohol, ether, acetone, ammonia, and acid. It is a compound with the typical crystal structure of the form MX2, exhibiting strong polarization effects.
- Chemical formula: CdI2
- Molar mass: 366.22 g/mol
- Appearance: white to pale yellow crystals
- Density: 5.640 g/cm3, solid
- Melting point: 387 °C (729 °F; 660 K)
- Boiling point: 742 °C (1,368 °F; 1,015 K)
- Solubility in water: 787 g/L (0 °C); 1250 g/L (100 °C)
- Solubility: soluble in ethanol, acetone, ether, and ammonia
- Crystal structure: Trigonal, hP3, space group P3m1, No. 164
- Coordination geometry: octahedral

Preparation
Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine.
CdO + 2HI → CdI2 + H2O
Also, the compound can be made by heating cadmium with iodine:
Cd +I2 → CdI2
A brownish crystalline β-form of the salt may be obtained by slow crystallization from solutions or fused salt mixtures.
It is used in lithography, photography, electroplating, process engraving, analytical chemistry, cadium electrodeposition, and the manufacture of phosphor and lubricants, among other things. It is created by reacting cadmium metal, or its oxide, hydroxide, or carbonate, with hydroiodic acid.
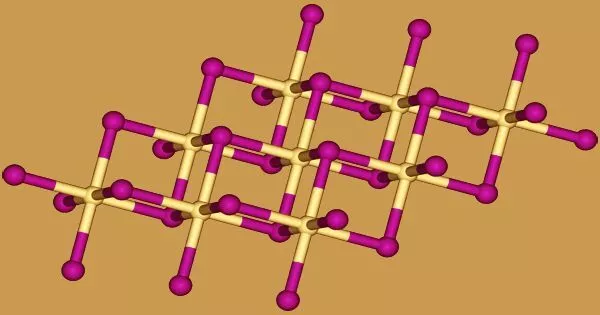
Crystal structure
The iodide anions form a hexagonal close-packed arrangement in cadmium iodide, while the cadmium cations fill all of the octahedral sites in alternate layers. The resulting structure is a layered lattice. Many other salts and minerals have the same basic structure. Cadmium iodide is mostly ionically bonded, but it also has some covalent properties.
Uses
The result of the action of hydriodic acid on cadmium oxide and crystallization. The colorless, flaky crystals are soluble in water, alcohol, and ether. This halide was a common iodizer for collodion formulas, particularly negatives. Cadmium iodide is used in lithography, photography, electroplating, and manufacturing phosphors, in the engraving process, and as an antiseptic and astringent.