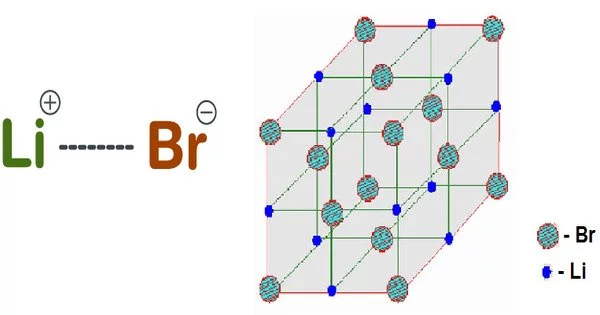
Lithium bromide (LiBr) is a lithium and bromine chemical compound. It is a lithium salt with bromide as the counterion. Because of its extreme hygroscopicity, LiBr can be used as a desiccant in certain air conditioning systems. The anhydrous salt, like common salt, forms cubic crystals. It is both a bromide and a lithium salt.
An ionic bond is formed in lithium bromide by the transfer of an electron from lithium to bromine. Because lithium donates an electron, it forms a cation and acquires the positive charge Li+. When bromine accepts an electron, it forms an anion and acquires the negative charge Br-. Lithium bromide has a cubic crystal structure.
Properties
Boiling point of lithium bromide is 12650C and melting point of lithium bromide is 5520C. It is a hygroscopic white solid. Its molar mass is 86.845 g/mol. Its density is 3.464 g/cm3. It is also soluble in methanol, ethanol, ether, acetone etc. It is slightly soluble in pyridine. It is not flammable.
- Chemical formula: LiBr
- Molar mass: 86.845 g/mol
- Appearance: White hygroscopic solid
- Density: 3.464 g/cm3
- Melting point: 550 °C (1,022 °F; 823 K)
- Boiling point: 1,300 °C (2,370 °F; 1,570 K)
- Solubility in water: 143 g/100 mL (0 °C); 266 g/100 mL (100 °C)
- Solubility: soluble in methanol, ethanol, ether, acetone, slightly soluble in pyridine
- Crystal structure: Cubic

Production
It is water, alcohol, and ether soluble. It is made by reacting hydrobromic acid with lithium hydroxide. On a small scale, it has been used for catalytic dehydrohalogenation in the synthesis of olefins. Lithium bromide aqueous solutions typically have low water vapour pressures.
LiBr is made by reacting lithium hydroxide with bromine or treating an aqueous suspension of lithium carbonate with hydrobromic acid. Unlike the other alkali metal bromides, it forms a number of crystalline hydrates.
Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water.
LiOH + HBr → LiBr + H2O
Uses
Desiccant in air-conditioning systems is a 50-60% aqueous solution of lithium bromide. Along with water, it is also used in absorption chilling (see absorption refrigerator). Solid LiBr is a useful organic synthesis reagent. It is used in oxidation and hydroformylation catalysts, as well as the deprotonation and dehydration of organic compounds containing acidic protons, as well as the purification of steroids and prostaglandins.
Applications in medicine
Beginning in the early 1900s, lithium bromide was used as a sedative, but it fell out of favor in the 1940s as newer sedatives became available, and after some heart patients died after using the salt substitute lithium chloride. It was used to treat bipolar disorder, just like lithium carbonate and lithium chloride.
Hazards
Lithium salts are both psychoactive and corrosive. Because lithium bromide has a negative enthalpy of solution, it generates heat quickly when dissolved in water.