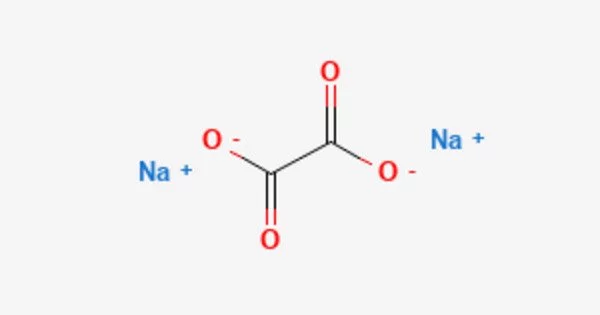
The sodium salt of oxalic acid with the formula Na2C2O4 is sodium oxalate, also known as disodium oxalate. It’s a white, crystalline, odorless solid that decomposes at temperatures above 290°C. Disodium oxalate can be used as a reducing agent and as a primary standard for standardizing potassium permanganate (KMnO4) solutions. Natroxalate is the mineral form of sodium oxalate. It is only found in extremely sodic ultra-alkaline pegmatites and is extremely rare.
It is a strong dicarboxylic acid found in a variety of plants and vegetables. It is produced in the body through the breakdown of glyoxylic acid or ascorbic acid. It is excreted in the urine rather than metabolized. It is a general reducing agent and an analytical reagent.
Properties
Sodium oxalate is an organic sodium salt consisting of sodium and oxalate ions in a 2:1 ratio. It has a role as a poison and a reducing agent. It is an oxalate salt and an organic sodium salt. It is an odorless white solid. Sinks and mixes slowly with water.
- Chemical formula: Na2C2O4
- Molar mass: 133.999 g mol−1
- Density: 2.34 g cm−3
- Melting point: 260 °C (500 °F; 533 K) decomposes above 290 °C
- Solubility in water: 2.69 g/100 mL (0 °C); 6.25 g/100 mL (100 °C)
- Solubility: soluble in formic acid; insoluble in alcohol, ether
- Crystal structure: monoclinic

Preparation
The neutralization of oxalic acid with sodium hydroxide (NaOH) in a 1:2 acid-to-base molar ratio yields sodium oxalate. Evaporation produces anhydrous oxalate, which can be thoroughly dried by heating to temperatures ranging from 200 to 250 °C.
In a 1:1 ratio, NaOH can be used to produce NaHC2O4, monobasic sodium oxalate, or sodium hydrogenoxalate. It can also be produced by decomposing sodium formate at temperatures above 360 °C.
Biological activity
Sodium oxalate, like several other oxalates, is toxic to humans. It can cause burning pain in the mouth, throat, and stomach, as well as bloody vomiting, headaches, muscle cramps, cramps and convulsions, a drop in blood pressure, heart failure, shock, coma, and death. The average lethal dose of oxalates is 10-15 grams/kilogram of body weight (per MSDS).
Like citrates, sodium oxalate can be used to remove calcium ions (Ca2+) from blood plasma. It also inhibits blood clotting. It should be noted that sodium oxalate can impair brain function and deposit calcium oxalate in the kidneys by removing calcium ions from the blood.