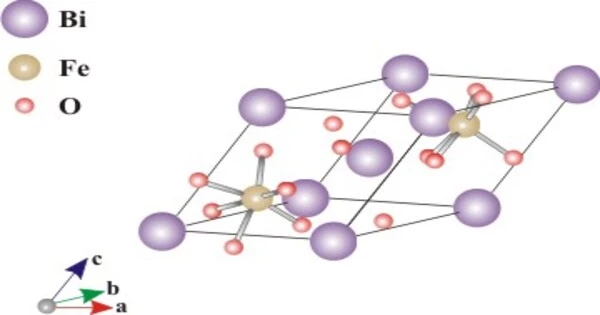
Bismuth ferrite (BiFeO3) is an inorganic chemical compound with a perovskite structure and an unusual compound of bismuth, iron, and oxygen (BFO). The room-temperature phase of BiFeO3 is rhombohedral and belongs to the space group R3c. It is one of the most promising lead-free piezoelectric materials with multiferroic properties at room temperature. It is synthesized in bulk and thin film forms, and both its antiferromagnetic (G type ordering) Néel temperature (approximately 653 K) and ferroelectric Curie temperature are well above room temperature (approximately 1100K).
Properties
- Compound Formula: BiFeO3
- Molecular Weight: 312.82
- Appearance: Crystalline solid
- Melting Point: 1255 °C
- Boiling Point: N/A
- Density: 8.22 g/cm3
- Solubility in H2O: N/A
- Crystal Phase / Structure: Perovskite
Sample Preparation
Bismuth ferrite is not a naturally occurring mineral and several synthesis routes to obtain the compound have been developed.
- Solid state synthesis
In the solid state reaction method, bismuth oxide (Bi2O3) and iron oxide (Fe2O3) are mixed in a mortar or by ball milling in a 1:1 mole ratio and then fired at high temperatures. Due to the volatility of bismuth during firing, which results in the formation of stable secondary Bi25FeO39 (selenite) and Bi2Fe4O9 (mullite) phases, preparing pure stoichiometric BiFeO3 is difficult. Typically, a firing temperature of 800 to 880 degrees Celsius is used for 5 to 60 minutes, followed by rapid cooling. Excess Bi2O3 has also been used to compensate for bismuth volatility and to prevent the formation of the Bi2Fe4O9 phase.
- Single crystal growth
Bismuth ferrite melts incongruently, but it can be grown from a bismuth oxide rich flux (e.g. a 4:1:1 mixture of Bi2O3, Fe2O3 and B2O3 at approximately 750-800 Celsius). High quality single crystals have been important for studying the ferroelectric, antiferromagnetic and magnetoelectric properties of bismuth ferrite.
- Chemical routes
To prepare phase pure BiFeO3, wet chemical synthesis routes based on sol-gel chemistry, modified Pechini routes, hydrothermal synthesis, and precipitation were used. The chemical routes have the advantage of precursor compositional homogeneity and reduced bismuth loss due to the much lower temperatures required.
The solution combustion reaction is a low-cost method for producing porous BiFeO3. To generate the reduction-oxidation (RedOx) reaction, a reducing agent (such as glycine, citric acid, urea, etc.) and an oxidizing agent (such as nitrate ions, nitric acid, etc.) are used. The appearance of the flame, and thus the temperature of the mixture, is determined by the ratio of oxidizing/reducing agents used.
- Thin films
In 2003, the electric and magnetic properties of high quality epitaxial thin films of bismuth ferrite were reported, reigniting scientific interest in the material. Epitaxial thin films have the significant advantage of being able to tune their properties through processing or chemical doping, as well as being able to be integrated into electronic circuitry. Epitaxial strain caused by singly crystalline substrates with different lattice parameters than bismuth ferrite can be used to change the crystal structure to monoclinic or tetragonal and thus change the ferroelectric, piezoelectric, or magnetic properties.
Applications
Being a room temperature multiferroic material and due to its ferroelectric photovoltaic (FPV) effect, bismuth ferrite has several applications in the field of magnetism, spintronics, photovoltaics, etc.
- Photovoltaics
A photocurrent is generated in a ferroelectric material under illumination in the FPV effect, and its direction is determined by the ferroelectric polarization of the material. As an alternative to traditional photovoltaic devices, the FPV effect has promising potential. However, due to their large bandgap and low conductivity, ferroelectric materials such as LiNbO3 generate very little photocurrent. Bismuth ferrite has shown great promise in this direction due to its large photocurrent effect and above bandgap voltage under illumination.