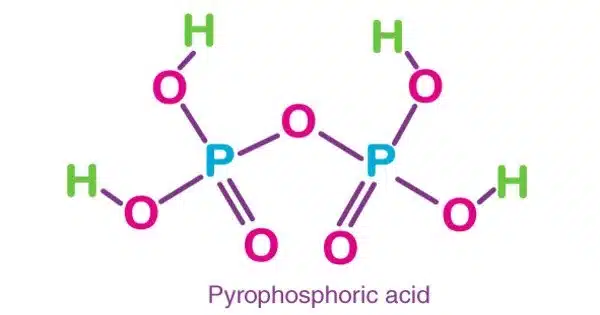
Pyrophosphoric acid, commonly known as diphosphoric acid, is an inorganic chemical with the formula H4P2O7 or, to put it another way, [(HO)2P(O)]2O. It has no color or flavor and is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes into two polymorphs that melt at 54.3 and 71.5 °C, respectively.
Polyphosphoric acid, a major source of phosphoric acid, contains the chemical. Pyrophosphates are anions, salts, and esters of pyrophosphoric acid. It is mostly employed in chemical processes as an intermediate or reagent. It is used in the pharmaceutical and chemical sectors. It can also be used as a dehydrator.
Properties
- Chemical Formula: H4P2O7
- Molecular Weight: 177.98 g/mol
- Appearance: It is a colorless, odorless, and viscous liquid at room temperature.
- Acidity: It is a strong acid and can donate two protons (H+) per molecule, making it a diprotic acid.
- Solubility: It is highly soluble in water, and its solutions are acidic.
- Stability: It is a relatively stable compound under normal conditions, but it can decompose at elevated temperatures.
Preparation
It can be prepared by reaction of phosphoric acid with phosphoryl chloride:
5 H3PO4 + POCl3 → 3 H4P2O7 + 3 HCl
It can also be made by ion exchange from sodium pyrophosphate or by processing lead pyrophosphate with hydrogen sulfide.
Boiling the water from orthophosphoric acid does not dehydrate it to pure pyrophosphoric acid; instead, a mixture of ortho, pyro, and polyphosphoric acids is produced; the maximum pyrophosphoric acid concentration remains below 50% and occurs slightly before what would otherwise be pure pyrophosphoric acid.
Application
The pyrophosphate ion is essential in metabolic reactions. It is involved in energy storage and transfer in cells, as well as a variety of metabolic activities such as DNA replication, signal transduction, and protein modification. Pyrophosphates are also employed in a variety of culinary products as an emulsifier, thickening agent, and stabilizer.
While pyrophosphoric acid has industrial use, it should be handled with caution because it is caustic and dangerous to human health. When working with any chemical, including pyrophosphoric acid and its derivatives, safety precautions must be taken.