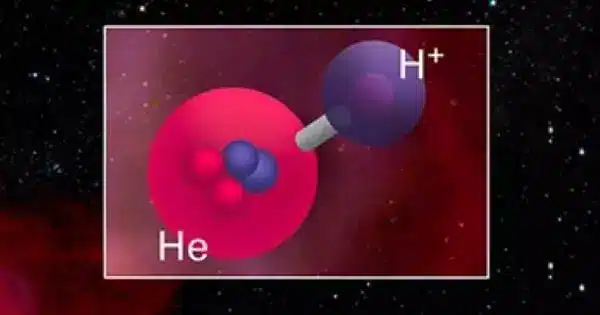
The helium hydride ion, also known as hydridohelium(1+) ion or helonium, is a positively charged cation with the chemical formula HeH+. It is made up of a helium atom bonded to a hydrogen atom that has had one electron removed. It is also known as protonated helium. It is the lightest heteronuclear ion and is thought to be the first compound formed after the Big Bang.
Helium hydride ions have been detected in a variety of astronomical environments, including planetary nebulae and H II regions with intense ultraviolet radiation. It’s also been found in the atmospheres of some exoplanets.
Properties
- Structure: The helium hydride ion has a linear structure, with the helium atom bonded to the hydrogen atom. The helium atom donates one of its electrons to the hydrogen atom, resulting in a single covalent bond between them.
- Bonding: The bonding in the helium hydride ion is primarily ionic in nature. The helium atom has a high ionization energy, and the hydrogen atom has a low electron affinity, making it favorable for helium to donate an electron to hydrogen.
- Stability: The helium hydride ion is stable in certain conditions, such as in the gas phase at low temperatures and in the presence of other ions or neutral atoms. However, it is highly reactive and can easily react with other molecules or ions.
- Electromagnetic Spectrum: The helium hydride ion has spectral lines in the far-infrared and submillimeter regions of the electromagnetic spectrum. These spectral lines are important for understanding the composition and physical conditions of various astronomical objects.
- Laboratory Synthesis: In laboratory settings, helium hydride ions can be produced by subjecting helium and molecular hydrogen to high-energy conditions, such as through electrical discharges or using particle accelerators.
The discovery and characterization of the helium hydride ion have provided important insights into the chemical processes that occurred in the early universe, as well as contributed to our understanding of molecular formation and evolution in astrophysical environments.
In 1925, the ion was created in a laboratory for the first time. It is stable in isolation, but extremely reactive, and cannot be prepared in bulk because it will react with any other molecule that comes into contact with it. The strongest known acid—stronger than even fluoroantimonic acid—its presence in the interstellar medium had been speculated about since the 1970s, and it was finally discovered in April 2019 using the airborne SOFIA telescope.