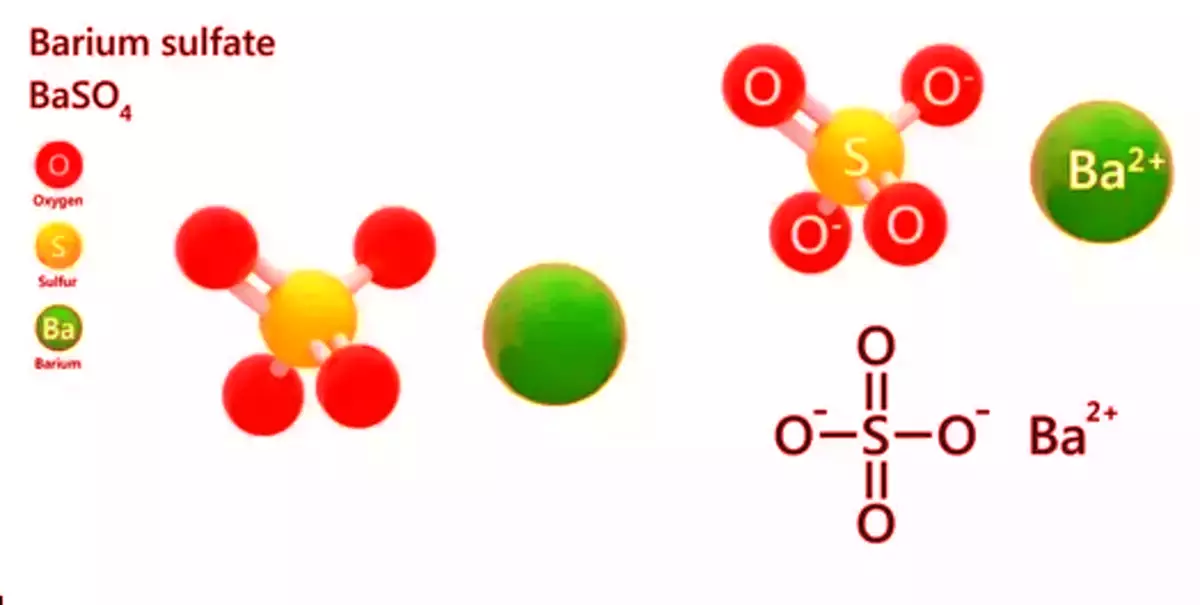
The inorganic compound barium sulfate (or sulphate) has the chemical formula BaSO. It appears as odorless white or yellowish powder or small crystals. It is a white crystalline solid that is odorless and water insoluble. It can be found in the mineral barite, which is the primary commercial source of barium and materials made from it. Its white opaque appearance and high density are used in its primary applications.
Because of its insolubility in water and radioopacity, barium sulfate has a wide range of medical and radio imaging applications. It is used in oil drilling as a weighting mud, as well as paints, paper coatings, linoleum, textiles, and rubber.
Properties
Pure barium sulfate is a white, odorless powder or small crystals with a density of 4.49 g/mL, a melting temperature of 1580 °C, and a boiling temperature of 1600 °C. It is said to be extremely insoluble in water, the universal solvent. In addition, it is insoluble in alcohols but soluble in concentrated acids. It has a strong reaction with aluminum powder.
- Chemical formula: BaSO4
- Molar mass: 233.39 g/mol
- Appearance: white crystalline
- Odor: odorless
- Density: 4.49 g/cm3
- Melting point: 1,580 °C (2,880 °F; 1,850 K)
- Boiling point: 1,600 °C (2,910 °F; 1,870 K) (decomposes)
- Solubility in water: 0.2448 mg/100 mL (20 °C); 0.285 mg/100 mL (30 °C)
- Solubility: insoluble in alcohol, soluble in concentrated, hot sulfuric acid

Production
Almost all of the barium consumed commercially is derived from barite, which is frequently highly impure. Barite is converted into barium sulfide through thermo-chemical sulfate reduction (TSR), also known as carbothermal reduction (heating with coke).
BaSO4 + 4 C → BaS + 4 CO
In contrast to barium sulfate, barium sulfide is soluble in water and easily converted to oxide, carbonate, and halides. To make highly pure barium sulfate, the sulfide or chloride is treated with sulfuric acid or sulfate salts:
BaS + H2SO → BaSO4 + H2S
Barium sulfate produced in this way is often called blanc fixe, which is French for “permanent white”. Blanc fixe is the form of barium encountered in consumer products, such as paints.
Barium sulfate is created in the laboratory by combining solutions of barium ions and sulfate salts. Due to the insolubility of barium sulfate, the least toxic salt of barium, wastes containing barium salts are sometimes treated with sodium sulfate to immobilize (detoxify) the barium. One of the most insoluble salts of sulfate is barium sulfate. Its low solubility is used as a test for Ba2+ ions as well as sulfate in qualitative inorganic analysis.
Uses
A contrast agent is barium sulfate. Barium sulfate works by coating the inside of your oesophagus, stomach, or intestines, making them more visible on a CT scan or other radiologic (x-ray) examination.
Barium sulfate may also be used for purposes not covered in this medication guide. Approximately 80% of the world’s barium sulfate production, mostly in the form of purified mineral, is used as a component of oil well drilling fluid. It raises the density of the fluid, raising the hydrostatic pressure in the well and decreasing the likelihood of a blowout.