Joule-Thomson effect was utilized to liquefy gases. When a highly compressed gas is allowed to expand through a vale or throttle into a region of low pressure so that no work is done against external pressure, the temperature of the gas falls. This is called the Joule- Thomson effect; after the famous porous-plug experiment of J. P. Joule ad W. Thomson (Lord Kelvin). For an ideal gas there should be no external work done when a gas expands from a higher pressure to a lower pressure. The cooling observed was, therefore, attributed to molecular attraction in real gases. When the gas expands the molecules get away from each other’s spheres of attractive forces. The energy necessary for this purpose is taken from the gas itself so that its temperature falls. Joule-Thomson effect is to be carefully distinguished from cooling by adiabatic expansion. Whereas in the former the expanding gas performs no external work, in adiabatic expansion the gas is made to do external work.
Under ordinary conditions H2 and He, however, show an increase of temperature in the Joule-Thomson experiment. If these gases were cooled below certain temperatures (e.g. – 80°C for H2 and – 240°C for He) the cooling effect was observed. In fact, for each gas there exists a temperature, known as the inversion temperature, above which the gas warms up when used in the Joule-Thomson experiment.
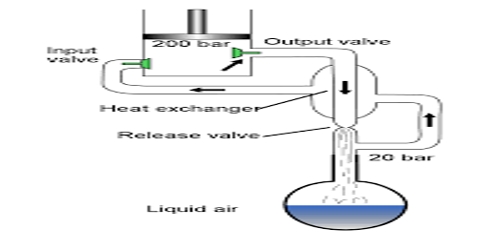
W. Hampson (1895) in England and C. von Linde (1895) in Germany employed the Joule-Thomson cooling effect for the manufacture of liquid air on a large scale. Of these the process of Linde is more widely used now-a-days because of its simplicity. (Figure) shows the apparatus of Uncle diagrammatically. The air to be liquefied is first freed from carbon dioxide and moisture as these, when condensed to solid, will block the apparatus. It is then cooled by passing through a freezing mixture, compressed to about 200 atmospheres and forced through the inner tube of the concentric pipes at A. These pipes are several hundred yards long and coiled spirally to save space.
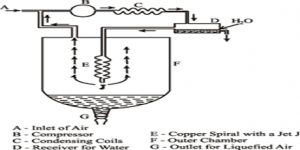
Fig: Linde apparatus for liquefaction of gases
The compressed air is allowed m expand suddenly into the vessel D to a pressure of about 50 atmospheres or less. The entry of the air into D is regulated by the throttle valve at T operated by the handle H.
This sudden expansion causes the temperature of the air to fall due to the Joule-Thomson effect. The cooled air passes through the annular space surrounding the inner tube A and thus cools the incoming compressed air. The temperature of this air on expansion through the valve T falls further. The expanded air, after passing through the annular space, is led to the compressor, again compressed to 200 atmospheres and then passed through the tube A. The process is repeated, the temperature of the air falling at each expansion step, and ultimately air is liquefied and collected in the vessel D. The tubes are all surrounded by insulating materials like wool, felt, feather etc in order to minimize absorption of heat from the surroundings
H2 and He were liquefied in quantity by applying the Joule- Munson effect, after being cooled to below their respective inversion temperatures Helium was first solidified at moderately high pressure in the Leyden cryogenic laboratory in Holland.