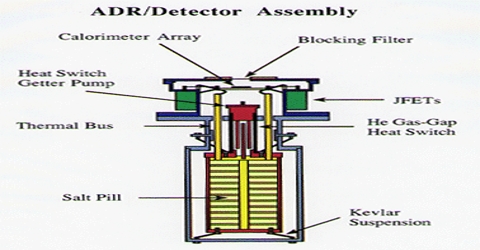
The principle of cooling by adiabatic demagnetization was suggested by P. Debye (1926) and W. F. Giauque (1927). When a substance is placed in a strong magnetic field, the magnetic dipoles are oriented or ordered. If the substance is insulated and the magnetic field is removed, the magnetic dipoles return to a random arrangement. Since the system is insulated the energy necessary for the return to the random state is taken from the system itself.
Adiabatic demagnetization is a process of cooling. It occurs in magneto-caloric materials. The principle is that these materials heat up when placed in a magnetic field and cool down when removed from the magnetic field.
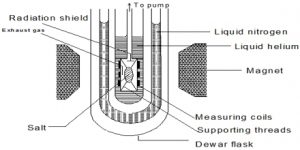
As a result the temperature falls. At ordinary temperatures the cooling effect of adiabatic demagnetization is not observed. But when the temperature is 1°K or less, the cooling effect becomes prominent. Salts of rare-earths, e.g. gadolinium, cerium and dysprosium, and ferric ammonium alum, potassium chrome alum etc. have been used in the cooling by adiabatic demagnetization process. Temperature as low as 0.0030 K has been achieved.