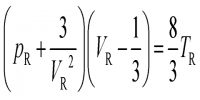Degree of dissociation
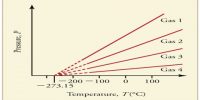
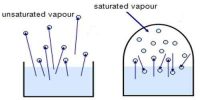
Measurement of the density of vapour may be used for calculating the degree of dissociation (α) of substances, i.e., the extent to which the substance dissociates. The degree of dissociation of a vapour may be defined as, ‘the fraction of the total number of molecules or moles which have dissociated’.
Consider 1 mole of the substance in the vapour and let α be the degree of dissociation or the fraction of 1 mole dissociated. Then (1 – α) moles will remain undissociated. If each of the dissociated molecules form n molecules of the gaseous products then a moles will yield nα moles of product. The total number of moles present in the vapour under the conditions of temperature is then
1 – α + nα;
or, 1 + (n – 1)α
If the pressure is kept constant then the volume occupied by the gas is directly proportional to the number of moles. If Va is the volume the vapour would have occupied if there was no dissociation. i.e., the value calculated from the ideal gas equation, and V is the volume actually occupied due to dissociation, then;
V/V0 = [1 + (n – 1)α] / 1 = ρ0/ρ … …. ….. (1)
since density, ρ, is inversely proportional to V. Here ρ0 = density of the vapour assuming no dissociation and ρ = observed density, both at the same pressure and temperature. The equation (1) may be rearranged to,
1 + (n – 1)α = ρ0/ρ
or, α = (ρ0 – ρ) / ρ(n-1)
Since the molecular mass is proportional to the density it follows that,
α = (M0 – M) / M(n-1) … …. ….. (2)
When, M0 is the molecular mass calculated from the formula;
Whets dissociation is studied in a closed vessel, i.e., at constant volume, the increase of pressure is inversely proportional to the density at constant temperature. So, one can also write;
P/P0 = 1 + (n – 1)α
so, α = (P-P0) / P0(n-1)… …. ….. (3)
where P and P0 are the pressures with and without dissociation.
Any of the above three equations (1), (2) and (3) may be used to determine the degree of dissociation of molecules upon heating.
Thermal dissociation is not accompanied by change in density or volume unless there is a change in the number of molecules due to dissociation.