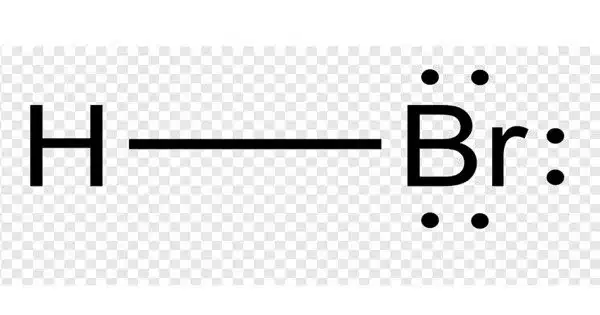The inorganic compound with the formula HBr is hydrogen bromide. It’s a hydrogen halide made up of hydrogen and bromine. It is a colorless gas that dissolves in water to form hydrobromic acid, which is saturated at room temperature at 68.85% HBr by weight. Aqueous solutions containing 47.6% HBr by mass form a constant-boiling azeotrope mixture with a boiling temperature of 124.3 °C. When less concentrated solutions are boiled, H2O is released until the constant-boiling mixture composition is reached.
Hydrogen bromide and its aqueous solution are common reagents used in the synthesis of bromide compounds. It is a highly soluble gas in water, where it reacts to form hydrobromic acid (HBr(aq)). At low temperatures, it can also exist as a liquid or solid.
Properties
- Chemical formula: HBr
- Molar mass: 80.91 g/mol
- Appearance: Colorless gas
- Odor: Acrid
- Density: 3.307 g/mL (25 °C)[2]
- Melting point: −86.9 °C (−124.4 °F; 186.2 K)
- Boiling point: −66.8 °C (−88.2 °F; 206.3 K)
- Solubility in water: 221 g/100 mL (0 °C); 130 g/100 mL (100 °C)
- Solubility: Soluble in alcohol, organic solvents
- Vapor pressure: 2.308 MPa (at 21 °C)
Industrial preparation
Hydrogen bromide (and hydrobromic acid) are created by combining hydrogen and bromine at temperatures ranging from 200 to 400 °C. Platinum or asbestos are commonly used to catalyze the reaction.
It is created by combining hydrogen gas (H2) and bromine gas (Br2). It can also be obtained as a byproduct of certain industrial processes, such as vinyl bromide production and the combustion of certain organic compounds.
Uses
Hydrogen bromide has various applications, including:
- Industrial Processes: It is used in the production of chemicals, such as pharmaceuticals, dyes, and flame retardants.
- Synthetic Organic Chemistry: It is used as a reagent in various organic reactions, including bromination reactions and the synthesis of specific compounds.
- Etching: It can be used as an etching agent in the semiconductor industry to remove thin layers of material.
- Cleaning: It can be used for cleaning purposes, particularly in the removal of oxide layers from metal surfaces.
Safety
Hydrogen bromide is a corrosive and toxic substance. It can cause severe burns and damage to the respiratory system upon inhalation. Proper precautions, such as working in well-ventilated areas and using personal protective equipment, should be taken when handling this compound. HBr is highly corrosive and, if inhaled, can cause lung damage.