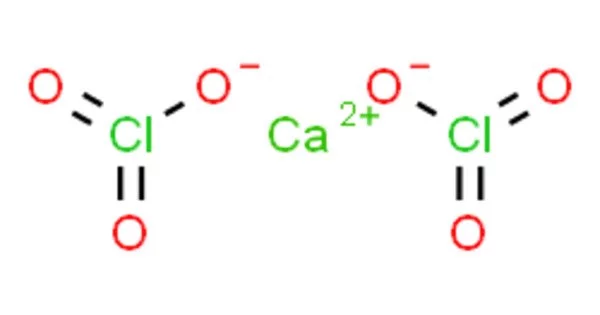
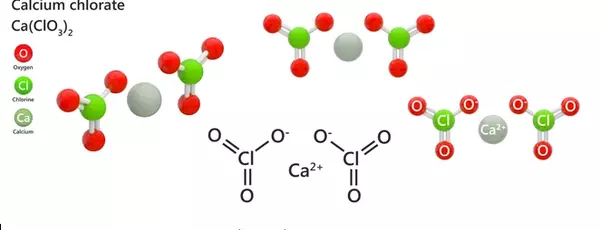
Calcium chlorate, with the chemical formula Ca(ClO3)2, is the calcium salt of chloric acid. It, like other chlorates, is a powerful oxidizer. It has the appearance of a white crystalline solid. It reacts with combustible materials to form a highly flammable mixture, which can be explosive if the combustible material is finely divided. Friction can ignite the mixture.
Strong sulfuric acid can cause fires or explosions if it comes into contact with it. When combined with ammonium salts, it is possible that spontaneous decomposition and ignition will occur. An explosion can occur if the material is exposed to fire or heat for an extended period of time. It has applications in photography, pyrotechnics, and as a herbicide.
Properties
The chemical compound formed by calcium and the chlorate anion is calcium chlorate Ca(ClO3)2. It is a strong oxidizer, similar to KClO3, and can be used in pyrotechnic formulations. It has a molecular mass of 206.98 g/mol. At 20°C, its solubility in water is 209 g/100 ml. It is only marginally soluble in alcohol. The melting point of calcium chlorate is 325°C, and its density is 2.71 g/cm3.
- Chemical formula: Ca(ClO3)2
- Molar mass: 206.98 g/mol
- Appearance: white solid, deliquescent
- Odor: odorless
- Density: 2.71 g/cm3
- Melting point: 150°C (dihydrate, decomp) 325°C
- Solubility in water: 209 g/100mL (20 °C); 197 g/100mL (25 °C)
- Crystal structure: monoclinic
Production
Calcium chlorate is made by passing chlorine gas through a hot suspension of calcium hydroxide in water, resulting in calcium hypochlorite, which disproportionates when heated with excess chlorine to produce calcium chlorate and calcium chloride:
6 Ca(OH)2 + 6 Cl2 → Ca(ClO3)2 + 5 CaCl2 + 6 H2O
This is also the first step in the Liebig process, which produces potassium chlorate.
In theory, electrolysis of hot calcium chloride solution yields chlorate, similar to the process used to produce sodium chlorate. In practice, electrolysis is complicated by calcium hydroxide deposits on the cathode, which prevents current flow.
Reactions
When concentrated solutions of calcium chlorate and potassium chloride are combined, potassium chlorate precipitates:
Ca(ClO3)2 + 2 KCl → 2 KClO3 + CaCl2
This is the second step of the Liebig process for the manufacture of potassium chlorate.
Solutions of calcium chlorate react with solutions of alkali carbonates to give a precipitate of calcium carbonate and the alkali chlorate in solution:
Ca(ClO3)2 + Na2CO3 → 2 NaClO3 + CaCO3
On strong heating, calcium chlorate decomposes to give oxygen and calcium chloride:
Ca(ClO3)2 → CaCl2 + 3 O2
Cold, dilute solutions of calcium chlorate and sulfuric acid react to give a precipitate of calcium sulfate and chloric acid in solution:
Ca(ClO3)2 + H2SO4 → 2 HClO3 + CaSO4
Because concentrated chloric acid is unstable, contact with strong sulfuric acid can result in explosions. In addition, contact with ammonium compounds can result in violent decomposition due to the formation of unstable ammonium chlorate.
Uses
Calcium chlorate, like sodium chlorate, has been used as a herbicide. It, like sodium chlorate, has been used as a herbicide. It is occasionally used in photography, poison ivy dusting powder, pyrotechnics, as an oxidizer, and as a pink flame colorant. Because of its hygroscopic nature and incompatibility with other common pyrotechnic materials (such as sulfur), its utility in these applications is limited.
Calcium chlorate is used as an oxidizer and pink flame colorant in pyrotechnics on occasion. Because of its hygroscopic nature and incompatibility with other common pyrotechnic materials (sulfur), its utility in these applications is limited.