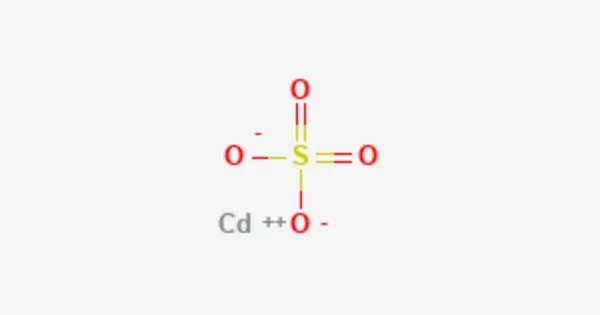
Cadmium sulfate is the name given to a group of inorganic compounds that have the formula CdSO4•xH2O. When heated, it produces toxic cadmium oxide fumes. The most common form is monohydrate CdSO4•H2O, but two other forms, CdSO4•83H2O and anhydrous salt, are also known (CdSO4). All salts are colorless and highly water soluble. It is a naturally occurring element found in the Earth’s crust. It is soft, ductile, and malleable, and it is usually found in the form of a mineral containing other elements.
Calcium sulfate is used in electroplating, vacuum tubes, fluorescent screens, analytical chemistry, as a fungicide, and as a chemical intermediate in the manufacture of cadmium pigments and stabilizers.
Properties
Cadmium sulfate is an odorless white solid. Sinks and mixes slowly with water. It’s molecular weight is 208.47 g/mol. Its density is 4.691 g/cm3. Its melting point is 1000οC. It’s boiling point is decomposes to basic sulfate and then the oxide.
- Chemical formula: CdSO4; CdSO4·H2O (monohydrate)
- Molar mass: 208.47 g/mol (anhydrous); 226.490 g/mol (monohydrate)
- Appearance: White hygroscopic solid
- Odor: odorless
- Density: 4.691 g/cm3 (anhydrous); 3.79 g/cm3 (monohydrate)
- Melting point: 1,000 °C (1,830 °F; 1,270 K) (anhydrous); 105 °C (monohydrate)
- Boiling point: (decomposes to basic sulfate and then oxide)
- Solubility in water: anhydrous: [75 g/100 mL (0 °C); 58.4 g/100 mL (99 °C)], monohydrate: 76.7 g/100 mL (25 °C)
- Solubility: slightly soluble in methanol, ethyl acetate; insoluble in ethanol

Preparation
X-ray crystallography shows that CdSO4·H2O is a typical coordination polymer. Each Cd2+ center has octahedral coordination geometry, being surrounded by four oxygen centers provided by four sulfate ligands and two oxygen centers from the bridging water ligands.
Cadmium sulfate hydrate can be prepared by the reaction of cadmium metal or its oxide or hydroxide with dilute sulfuric acid:
CdO + H2SO4 → CdSO4 + H2O
Cd + H2SO4 → CdSO4 + H2
The anhydrous material can be prepared using sodium persulfate:[citation needed]
Cd + Na2S2O8 → CdSO4 + Na2SO4
Occurrence
Cadmium sulfates occur as the following rare minerals drobecite (CdSO4·4H2O), voudourisite (monohydrate), and lazaridisite (the 8/3-hydrate).
Applications
Cadmium sulfate is widely used for cadmium electroplating in electronic circuits. It’s also a precursor to cadmium pigments like cadmium sulfide. It’s also used as an electrolyte in a Weston standard cell and a pigment in fluorescent screens.
Calcium sulfate can also be used as a fungicide in vacuum tubes, fluorescent screens, and analytical chemistry. Also used as a chemical intermediate in the production of cadmium pigments and stabilizers. Calcium sulfate is a known carcinogen that has been linked to an increased risk of developing lung cancer.