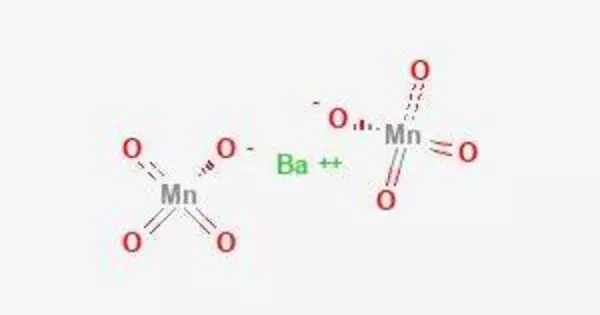
Barium permanganate (Ba(MnO4)2) is a chemical compound with the formula Ba(MnO4)2. It is generated via the reaction of barium (Ba) cations and permanganate (MnO4-) anions. It generates violet to brown crystals that are very marginally soluble in water. Permanganate is a -1 charged anion produced from manganese(VII) oxide. Barium is a metal in the alkaline earth metal group with a charge of +2 when it forms compounds.
Barium permanganate, like other permanganate compounds, is a powerful oxidizing agent. It is less typically seen than potassium permanganate, the more well-known permanganate chemical utilized in a variety of applications, including as an oxidizing agent, disinfectant, and in science laboratories.
Properties
Barium permanganate is typically found as dark purple crystals or a purple powder. It is sparingly soluble in water. The density of barium permanganate can vary, but it is generally in the range of 3.8 to 4.0 g/cm³. It has a relatively high melting point, typically around 300-350°C (572-662°F). It is a powerful oxidizing agent, like other permanganates. It can react vigorously with reducing agents, causing combustion or explosive reactions.
- Chemical formula: Ba(MnO4)2
- Molar mass: 375.198 g/mol
- Appearance: dark violet to brown crystals
- Odor: odorless
- Density: 3.77 g/cm3
- Melting point: 200 °C (392 °F; 473 K) (decomposes)
- Solubility in water: 62.5 g/100 mL (29 °C)
- Solubility: decomposes in alcohol
- Crystal structure: rhombic
Preparation
Barium permanganate may be produced by disproportionation of barium manganate in a mildly acidic solution, including solutions carbon dioxide or sulfuric acid:
3 BaMnO4 + 2 CO2 → Ba(MnO4)2 + 2 BaCO3 + MnO2
3 BaMnO4 + 2 H2SO4 → Ba(MnO4)2 + 2 BaSO4 + MnO2 + 2 H2O
It is also possible to make it by oxidizing barium manganate using powerful oxidants. Preparations based on aqueous reactions of barium manganate are extremely sluggish due to the manganate’s low solubility.
Another method for producing barium permanganate is through the interaction of silver permanganate and barium chloride. Highly pure samples can be made using a similar reaction between potassium permanganate and aluminum sulfate, which results in aluminum permanganate, which is then reacted with a stoichiometric quantity of barium hydroxide.
Application
Like other permanganates, barium permanganate can be employed in oxidation reactions and certain chemical processes. However, due to barium’s toxicity, its use may be limited, and it is not as widely utilized as other permanganate compounds.
Safety Considerations
Permanganates are powerful oxidizers and barium compounds are hazardous. Handling barium permanganate should be done with caution, and proper safety precautions should be followed to avoid skin or eye contact, as well as ingestion or inhalation.