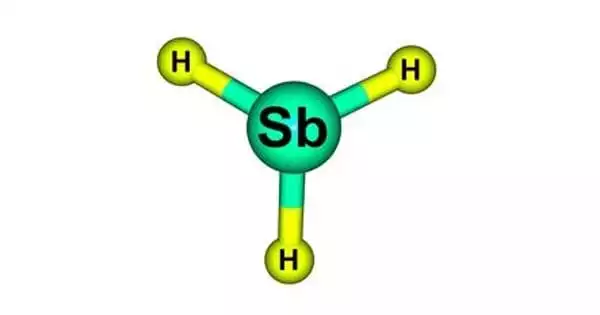
The chemical compound stibine has the formula SbH3. It is a colorless, highly toxic gaseous chemical. This colorless gas, a pnictogen hydride, is the main covalent hydride of antimony and a heavy homologue of ammonia. The molecule is pyramidal, with 91.7° H–Sb–H angles and 170.7 pm (1.707Å) Sb–H lengths. This gas smells strongly of hydrogen sulfide (rotten eggs).
Properties
Stibine appears as a colorless gas with a disagreeable odor. It is a moderate fire hazard that may yield toxic fumes when heated above 392°F. It is irritating to the skin, eyes, and mucous membranes. It is heavier than air. If exposed to prolonged fire or intense heat, the container may rupture violently or rocket.
- Chemical formula: SbH3
- Molar mass: 124.784 g/mol
- Appearance: Colourless gas
- Odor: unpleasant, like hydrogen sulfide
- Density: 5.48 g/L, gas
- Melting point: −88 °C (−126 °F; 185 K)
- Boiling point: −17 °C (1 °F; 256 K)
- Solubility in water: slightly soluble
- Solubility in ethanol: soluble
Chemical Properties
The chemical properties of SbH3 resemble those for AsH3. Typical for a heavy hydride (e.g. AsH3, H2Te, SnH4), SbH3 is unstable with respect to its elements. The gas decomposes slowly at room temperature but rapidly at 200 °C:
2 SbH3 → 3 H2 + 2 Sb
The decomposition is autocatalytic and can be explosive.
SbH3 is readily oxidized by O2 or even air:
2 SbH3 + 3 O2 → Sb2O3 + 3 H2O
SbH3 exhibits no basicity, but it can be deprotonated:
SbH3 + NaNH2 → NaSbH2 + NH3

Preparation
SbH3 is generally prepared by the reaction of Sb3+ sources with H− equivalents:
2 Sb2O3 + 3 LiAlH4 → 4 SbH3 + 1.5 Li2O + 1.5 Al2O3
4 SbCl3 + 3 NaBH4 → 4 SbH3 + 3 NaCl + 3 BCl3
Alternatively, sources of Sb3− react with protonic reagents (even water) to also produce this unstable gas:
Na3Sb + 3 H2O → SbH3 + 3 NaOH
Uses
Stibine is used in the semiconductor industry to dope silicon with minuscule amounts of antimony using the chemical vapour deposition procedure (CVD). It has also been employed in epitaxial layers as a silicon dopant. SbH3 has been reported to be used as a fumigant, however its instability and difficult preparation contrast with the more usual fumigant phosphine.
History
Because stibine (SbH3) is identical to arsine (AsH3), the Marsh test detects it as well. This sensitive test detects arsine produced in the presence of arsenic. This process, invented about 1836 by James Marsh, involves treating a sample with arsenic-free zinc and dilute sulfuric acid: if the sample contains arsenic, gaseous arsine will arise. The gas is drawn into a glass tube and decomposed by heating to roughly 250 – 300 °C. The presence of arsenic is indicated by the creation of a deposit in the heated area of the equipment. The production of a black mirror deposit in the cold area of the equipment shows the presence of antimony.
Lewis Thomson and Pfaff discovered stibine independently in 1837. It took some time before the properties of the toxic gas could be determined, partly because a suitable synthesis was not available. In 1876 Francis Jones tested several synthesis methods, but it was not before 1901 when Alfred Stock determined most of the properties of stibine.
Safety
SbH3 is an unstable flammable gas. It is highly toxic, with an LC50 of 100 ppm in mice.
Toxicology
For the toxicology of other antimony compounds, see Antimony trioxide.
The toxicity of stibine is distinct from that of other antimony compounds, but similar to that of arsine. Stibine attaches to the haemoglobin of red blood cells, leading the body to eliminate them. Although animal studies indicate that their toxicities are equal, most occurrences of stibine poisoning have been accompanied with arsine poisoning. The first symptoms of exposure are headaches, vertigo, and nausea, which can take several hours to appear, followed by hemolytic anemia (high levels of unconjugated bilirubin), hemoglobinuria, and nephropathy.