Beryllium iodide is an inorganic compound with the formula BeI2. It is a white hygroscopic solid. It is a beryllium iodide. Beryllium is an atomic number 4 lightweight alkaline earth metal. It is a relatively uncommon element that occurs naturally only in combination with other elements in minerals.
Properties
Beryllium iodide, when sublimed, is composed of colorless crystals that decompose quickly in moist air. At 15°C, its specific gravity is close to 4.20 g/cm3. It starts to sublime below its melting point of 510°C. The boiling point of melted iodide is between 585 and 595°C. It is insoluble in benzene, toluene, turpentine spirits, and only slightly soluble in carbon disulphide. The slightest trace of water attacks it immediately, but if it is first fused, its sensitivity to water is much less. It can be distilled without alteration in dry hydrogen, nitrogen or carbon dioxide.
- Molar mass: 262.821 g/mol
- Appearance: colorless needle-like crystals
- Density: 4.325 g/cm3
- Melting point: 480 °C (896 °F; 753 K)
- Boiling point: 590 °C (1,094 °F; 863 K)
- Solubility in water: reacts with water
- Solubility: Slightly soluble in CS2
- Crystal structure: orthorhombic
Reactions
Beryllium iodide can be prepared by reacting beryllium metal with elemental iodine at temperatures of 500°C to 700°C:
Be + I2 → BeI2
Beryllium iodide is also formed when beryllium carbide reacts with hydrogen iodide in the gas phase:
Be2C + 4 HI → 2 BeI2 + CH4
Beryllium iodide reacts with fluorine to produce beryllium fluoride and iodide fluorides, with chlorine to produce beryllium chloride, and with bromine to produce beryllium bromide.
The iodine in beryllium iodide is easily replaced by other halogens; it reacts with fluorine to produce beryllium fluoride and fluorides of iodine, with chlorine to produce beryllium fluoride, and with bromine to produce beryllium bromide. Both the solid and the vapor are flammable in air.
Preparation
Beryllium iodide has the formula BeI2. It is very hygroscopic and reacts violently with water, forming hydroiodic acid. Beryllium iodide can be prepared by reacting Be metal with elemental iodine at temperatures of 500°C–700°C:
Be+I2→BeI2
Beryllium iodide is also formed when beryllium carbide reacts with hydrogen iodide in the gas phase:
Be2C+4HI→2BeI2+CH4
Wahler (1828) and Debray (1855) were the first to prepare Beryllium Iodide, by the action of iodine on the metal. Lebeau (1898) was the first to prepare large amounts (for the time) of carbide by the action of gaseous hydroiodic acid, or a mixture of hydrogen and iodine vapor, on the carbide at around 700°C.
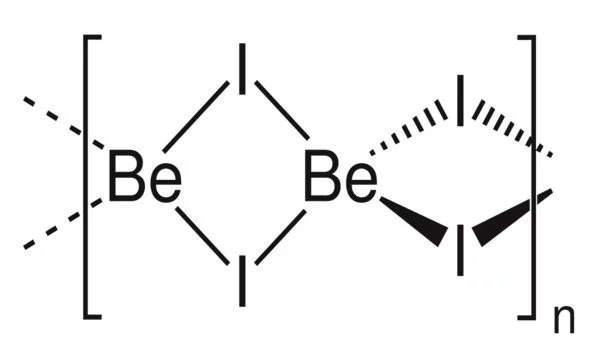
Structure
BeI2 exists in two forms (polymorphs). Both structures are made up of tetrahedral Be2+ centers linked together by doubly bridging iodide ligands. Edge-sharing polytetrahedra are one form. The other form has interconnected adamantane-like cages that resemble zinc iodide.
Applications
Beryllium iodide can be used in the preparation of high-purity beryllium by decomposing the compound on a hot tungsten filament.