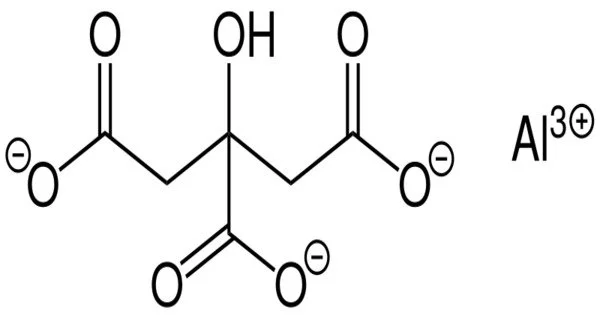
Aluminium citrate is a chemical compound with the chemical formula AlC6H5O7. This white, crystalline salt is produced by mixing aluminium chloride hexahydrate with citric acid. It is a compound made up of aluminum cations and citrate anions. It is a compound that is formed from the reaction of citric acid and aluminum hydroxide.
Aluminum citrate is a compound made up of aluminum ions and citrate ions. It is used as an ingredient in antacids and as a sequestrant in cosmetics and personal care products. In the body, aluminum citrate can bind to bile acids and help reduce the amount of cholesterol absorbed from the gut. In some cases, it is used as an antacid to treat acid indigestion and heartburn.
Properties
It can be manufactured with aluminum chloride and citric acid as the starting materials. The pH of the mixture can be adjusted with either ammonium hydroxide or sodium hydroxide to obtain aluminum citrate.
- Chemical formula: AlC6H5O7
- Molar mass: 216.08 g/mol
- Appearance: White solid
- Solubility in water: Insoluble

Uses
Aluminum citrate can be used as a crosslinker for many polymers in the oil industry. It is commonly used as an ingredient in antacids to neutralize excess stomach acid, as well as in certain cosmetics as a source of aluminum. It is also used as an antiperspirant.
Effects on humans
Aluminium citrate absorbs approximately 8% of the aluminum in blood due to the ability of Al3+ ions to replace Ca2+ from calcium citrate and is known to cause chronic renal failure due to an increase in phosphorus in the kidneys. It is suspected of causing Alzheimer’s disease, but more research is needed. This compound may also have some beneficial effects on humans, including the prevention of silicosis. When ingested, 80% of the compound is excreted through the body via urine, with the remainder passing more slowly.