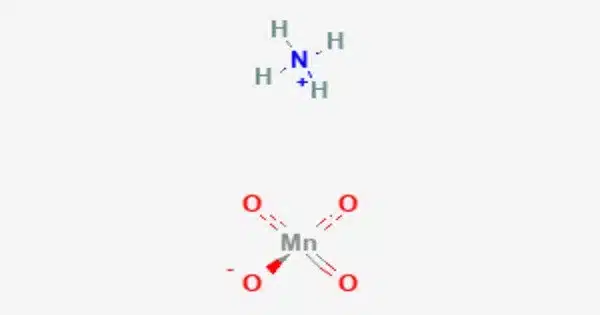
Ammonium permanganate is the chemical compound NH4MnO4, also known as NH3•HMnO4. It is a water-soluble violet-brown or dark purple salt. It is a chemical compound that contains ammonium ions (NH4+) and permanganate ions (MnO4-). Ammonium permanganate, like other permanganate compounds, has strong oxidizing characteristics. It is mostly utilized as an oxidizing agent in numerous chemical processes.
Preparation
Ammonium permanganate was first prepared by Eilhard Mitscherlich in 1824 by reaction of silver permanganate with equal molar amount of ammonium chloride, filtering the silver chloride and evaporating the water.
AgMnO4 + NH4Cl → AgCl + NH4MnO4
It can also be prepared in a similar way from potassium permanganate and ammonium chloride.
KMnO4 +NH4Cl → KCl + NH4MnO4
Properties
- Chemical formula: NH4MnO4
- Molar mass: 136.974 g/mol
- Appearance: rhombic needle crystals or powder with rich violet-brown or dark purple metallic sheen, become steel-gray in storage; magenta–rose in solution
- Density: 2.2g/cm3, solid
- Melting point: decomposes
- Solubility in water: 8.0 g/100 ml at 15 °C
- Crystal structure: Orthorhombic
Stability
Ammonium permanganate is not very stable and can disintegrate over time, especially in the presence of moisture. This decomposition may produce ammonia gas, thus it should be stored in a dry atmosphere and handled with caution.
Ammonium permanganate is a strong oxidant due to its permanganate anion, and it is a moderately strong explosive due to the combination of oxidizer permanganate anion and reducing ammonium cation. Dry ammonium permanganate can detonate due to heat, shock, or friction, and it can explode at temperatures exceeding 140 °F (60 °C).
Ammonium permanganate decomposes explosively to manganese dioxide, nitrogen, and water:
2 NH4MnO4 → 2 MnO2 + N2 + 4 H2O
Ammonium permanganate decomposes slowly in storage even at normal temperatures. A sample stored for 3 months was only 96% pure, after 6 months it assumed the color of iodine and had a strong smell of nitrogen oxides. It emits toxic fumes when decomposed by heat.
Uses
While ammonium permanganate (AP) is not as widely utilized as potassium permanganate (KMnO4), it has applications in some chemical processes and laboratory investigations that require a less commonly accessible oxidizing agent. It can be used for organic synthesis, analytical chemistry, and water treatment, among other things.
Safety
Like other permanganates, ammonium permanganate should be handled with caution. It is a powerful oxidant that can cause intense reactions with some organic and flammable compounds. When working with this substance, proper safety precautions, including protective equipment, should be taken.