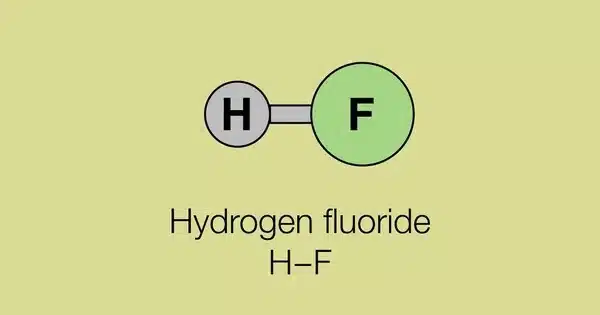
Fluorane (hydrogen fluoride) is an inorganic compound with the chemical formula HF. It is a chemical compound composed of hydrogen and fluorine. It is a highly toxic, colourless gas or liquid that dissolves in water to form an aqueous solution known as hydrofluoric acid. It is the primary industrial source of fluorine, often in the form of hydrofluoric acid, and serves as a key feedstock in the production of a wide range of important compounds, including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE).
At room temperature, it is a colourless gas, but depending on temperature and pressure, it can also exist as a colourless liquid or a white crystalline solid. HF has a pungent odour and is highly corrosive and toxic.
Properties
- Chemical formula: HF
- Molar mass: 20.006 g·mol−1
- Appearance: colourless gas or colourless liquid (below 19.5 °C)
- Odor: unpleasant
- Density: 1.15 g/L, gas (25 °C); 1.663 g/mL, solid (–125 °C)
- Melting point: −83.6 °C (−118.5 °F; 189.6 K)
- Boiling point: 19.5 °C (67.1 °F; 292.6 K)
- Solubility in water: completely miscible (liquid)
- Vapor pressure: 783 mmHg (20 °C)
- Acidity (pKa): 3.17 (in water), 15 (in DMSO)
- Conjugate acid: Fluoronium
- Conjugate base: Fluoride
Occurrence
Hydrogen fluoride is rarely found in its pure form in nature. It can be made by reacting calcium fluoride (CaF2) with sulfuric acid (H2SO4) or fluorspar (CaF2) with concentrated sulfuric acid. HF is not regarded as a significant polluter of the environment. However, releasing it into water or soil can have a negative impact on aquatic life and ecosystems.
Application
HF is used in a number of industrial processes. It is widely used in the manufacture of aluminium, fluorocarbons, and other fluorine-containing chemicals. It is also used in the semiconductor industry as an etching agent and as a catalyst in certain chemical reactions. HF is also widely used as a component of superacids in the petrochemical industry. It boils at near room temperature, much higher than other hydrogen halides, due to strong and extensive hydrogen bonding.
Safety Considerations
Hydrogen fluoride is extremely corrosive and poses serious health risks. When it comes into contact with the skin, eyes, or respiratory system, it can cause severe burns. Inhaling or ingesting HF can cause internal damage such as lung injury and bone damage. When working with HF or its solutions, proper safety precautions, such as protective clothing and equipment, are required.