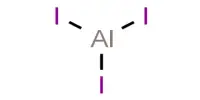
Aluminium chloride (AlCl3) refers to compounds with the formula AlCl3(H2O)2 (n = 0 or 6). It has a pungent odor and appears as a white to gray powder. They are made up of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six hydration fluids. Both are white solids, but samples are frequently contaminated with iron(III) chloride, which causes them to turn yellow. Aluminium chloride is typically whitish in appearance. However, because of the presence of impurities (iron(III) chloride), it turns yellowish.
Commercially, anhydrous material is significant. It has a low melting point and a high boiling point. It is primarily manufactured and consumed in the production of aluminum metal, although it is also utilized extensively in other sectors of the chemical industry. The chemical is frequently referred to as a Lewis acid. It is an example of an inorganic chemical that, at low temperatures, reversibly converts from a polymer to a monomer.
Properties
The melting and boiling points of aluminum chloride are quite low. It reaches its pinnacle at 180°C. Aluminium chloride is white in color, but it is frequently contaminated by iron trichloride, which turns it yellow. Only at pressures greater than 2.5 atm and temperatures greater than 190°C does it exist as a liquid.
- Molar Mass: 133.341 g/mol (anhydrous) and 241.432 g/mol (hexahydrate)
- Density: 2.48 g/cm3 (anhydrous) and 2.398 g/cm3 (hexahydrate)
- Melting Point: 192.6°C (anhydrous) and 100°C (hexahydrate, dec.)
- Boiling Point: 180°C
Preparation
Aluminium chloride is mainly produced using an exothermic reaction of two elements namely aluminium and chlorine. There are several other ways in which aluminium chloride can be obtained.
Some common ways are by reacting the aluminium metal with hydrogen chloride or by conducting a single displacement reaction between copper chloride and aluminium metal. The reactions for the same are given below:
2Al + 3Cl2 → 2AlCl3
2Al + 6HCl → 2AlCl3 + H2
2Al + 3CuCl2 → 2AlCl3 + 3Cu
Synthesis
Aluminium chloride is employed in the manufacturing of aluminum metal, but it also has several applications in the chemical industry, particularly as a Lewis acid. Aluminium chloride (AlCl3) is covalently linked and has a low melting and boiling point.
Aluminium chloride is produced on a large scale through an exothermic reaction of aluminium metal with chlorine or hydrogen chloride at temperatures ranging from 650 to 750 °C (1,202 to 1,382 °F).
2 Al + 3 Cl2 → 2 AlCl3
2 Al + 6 HCl → 2 AlCl3 + 3 H2
Aluminum chloride may be formed via a single displacement reaction between copper chloride and aluminum metal.
2 Al + 3 CuCl2 → 2 AlCl3 + 3 Cu
In the US in 1993, approximately 21,000 tons were produced, not counting the amounts consumed in the production of aluminium.
By dissolving aluminium oxides with hydrochloric acid, hydrated aluminium trichloride is produced. Metallic aluminum easily dissolves in hydrochloric acid, releasing hydrogen gas and producing a significant amount of heat. When heated, this material does not form anhydrous aluminium trichloride; instead, the hexahydrate decomposes to aluminium hydroxide:
Al(H2O)6Cl3 → Al(OH)3 + 3 HCl + 3 H2O
Aluminium also forms a lower chloride, aluminium(I) chloride (AlCl), but this is very unstable and only known in the vapour phase.
Uses
AlCl3 is primarily utilized as a catalyst in a variety of chemical processes. It is widely employed in the Friedel-Crafts process, which includes both acylations and alkylations. It’s employed in the synthesis of anthraquinone from phosgene and benzene.
Aldehyde groups on aromatic series or rings can be brought in or attached using aluminium chloride. Consider the Gatterman-Koch reaction, in which the Lewis acid (aluminum chloride) is employed to remove a chloride ion from the species.
It is also employed in the polymerization and isomerization of low molecular weight hydrocarbons. One common example is the manufacture of dodecylbenzene for detergents.
Safety
Anhydrous AlCl3 reacts vigorously with bases, so suitable precautions are required. It can cause irritation to the eyes, skin, and respiratory system if inhaled or on contact.