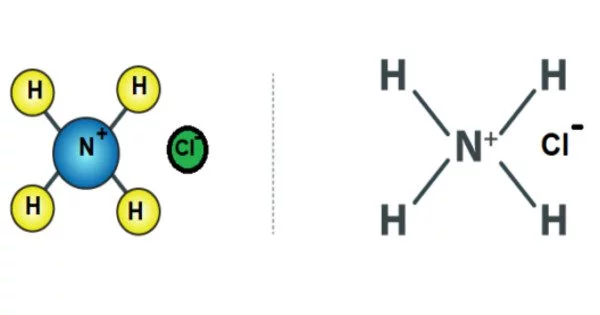
Ammonium chloride is a white crystalline salt that is highly soluble in water. It is an inorganic compound with the formula NH4Cl. It is a byproduct of the production of sodium carbonate. Ammonium chloride solutions are mildly acidic. It is known as sal ammoniac in its naturally occurring mineralogic form. The mineral is commonly formed by condensation of coal-derived gases on burning coal dumps. It is also found near certain types of volcanic vents. It is primarily used as a fertilizer and as a flavoring agent in certain types of liquorice. It is the result of a reaction between hydrochloric acid and ammonia.
Its primary applications are as a nitrogen source in fertilizers and as an electrolyte in dry cells, but it is also widely used as a component of galvanizing, tinning, and soldering fluxes to remove oxide coatings from metals and thus improve solder adhesion.
Properties
Ammonium chloride is a crystalline substance that is colorless. It is highly soluble in water, forming a slightly acidic solution quickly. At 340 °C (644 °F), it vaporizes without melting, forming equal parts ammonia and hydrogen chloride. It is produced as a byproduct of the ammonia-soda process used to produce sodium carbonate.
- Chemical formula: ClH4N
- Molar mass: 53.49 g·mol−1
- Appearance: White solid, hygroscopic
- Odor: Odorless
- Density: 1.519 g/cm3
- Solubility in water: 294 g/L (0 °C); 383.0 g/L (25 °C); 740.8 g/L (100 °C)
- Solubility product (Ksp): 30.9 (395 g/L)
- Solubility: Soluble in liquid ammonia, hydrazine,
- Slightly soluble in acetone
- Insoluble in diethyl ether, ethyl acetate

Production
Ammonium chloride is found naturally in volcanic areas, where it forms on volcanic rocks near fume-emitting vents (fumaroles). The crystals form directly from the gaseous state and have a short lifetime because they dissolve easily in water.
It is a product of the Solvay process used to produce sodium carbonate:
CO2 + 2 NH3 + 2 NaCl + H2O → 2 NH4Cl + Na2CO3
Not only is that method the principal one for the manufacture of ammonium chloride, but also it is used to minimize ammonia release in some industrial operations.
Ammonium chloride is prepared commercially by combining ammonia (NH3) with either hydrogen chloride (gas) or hydrochloric acid (water solution):
NH3 + HCl → NH4Cl
Preparation
Ammonium chloride is prepared commercially by reaction of ammonia (NH3) with hydrogen chloride.
NH3 + HCl → NH4Cl
Ammonium chloride is also formed as the by-product of the Solvay Process. In this process carbon dioxide and ammonia are passed into a cold saturated solution of sodium chloride.
CO2 + 2 NH3 + 2 NaCl + H2O → 2 NH4Cl + Na2CO3
Reactions
Ammonium chloride appears to sublime upon heating but actually decomposes into ammonia and hydrogen chloride gas:
NH4Cl → NH3 + HCl
Ammonium chloride reacts with a strong base, like sodium hydroxide, to release ammonia gas:
NH4Cl + NaOH → NH3 + NaCl + H2O
Similarly, ammonium chloride also reacts with alkali-metal carbonates at elevated temperatures, giving ammonia and alkali-metal chloride:
2 NH4Cl + Na2CO3 → 2 NaCl + CO2 + H2O + 2 NH3
A solution of 5% by mass of ammonium chloride in water has a pH in the range 4.6 to 6.0.
Some of ammonium chloride’s reactions with other chemicals are endothermic, like its reaction with barium hydroxide and its dissolving in water.
Applications
The most common application of ammonium chloride is as a nitrogen source in fertilizers such as chloroammonium phosphate (which accounts for 90% of global ammonium chloride production). In Asia, the main crops fertilized this way are rice and wheat.
In the 18th century, ammonium chloride was used in pyrotechnics, but it was eventually replaced by safer, less hygroscopic chemicals. Its purpose was to act as a chlorine donor, enhancing the green and blue colors produced by the copper ions in the flame. It had a secondary use as a white smoke producer, but its ready double decomposition reaction with potassium chlorate produced the highly unstable ammonium chlorate, making its use extremely hazardous.
Because of its expectorant properties, it is used to prevent urinary stones in goats, cattle, and sheep, and it is a component of many proprietary cold and cough remedies.
Health Consequences
Overdosing is a possibility, so it’s not completely risk-free. Ammonium chloride can cause high blood pressure. Ammonium chloride poisoning causes irritation, shortness of breath, coughing, nausea, and headache. The gases have the potential to cause severe eye irritation. Chronic exposure can cause asthma-like symptoms or impair kidney function.