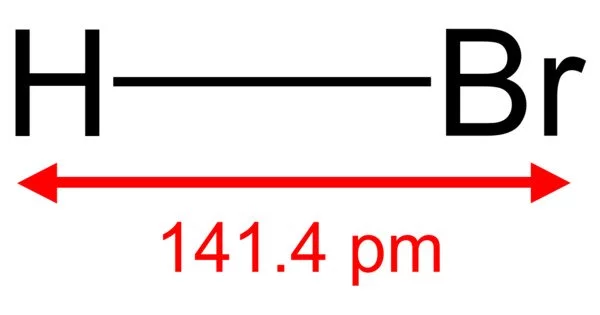
The inorganic substance with the formula HBr is hydrogen bromide. It is a hydrogen halide made up of both bromine and hydrogen. It is a colorless gas that, when dissolved in water, produces hydrobromic acid, which, at room temperature, is saturated with 68.85% HBr by weight. A constant-boiling azeotrope mixture made up of aqueous solutions containing 47.6% HBr by mass boils at 124.3 °C. Up until the constant-boiling mixture composition is obtained, boiling less concentrated solutions releases H2O.
The creation of bromide compounds frequently uses the reagents hydrogen bromide and its aqueous solution.
Properties
- Chemical formula: HBr
- Molar mass: 80.91 g/mol
- Appearance: Colorless gas
- Odor: Acrid
- Density: 3.307 g/mL (25 °C)
- Melting point: −86.9 °C (−124.4 °F; 186.2 K)
- Boiling point: −66.8 °C (−88.2 °F; 206.3 K)
- Solubility in water: 221 g/100 mL (0 °C); 130 g/100 mL (100 °C)
- Solubility: Soluble in alcohol, organic solvents
- Conjugate acid: Bromonium
- Conjugate base: Bromide
- Molecular shape: Linear
Reactions
Organic chemistry
Hydrogen bromide and hydrobromic acid are important reagents in the production of organobromine compounds. In a free-radical reaction, HBr adds to alkenes:
RCH=CH2 + HBr → R−CHBr−CH3
The resultant alkyl bromides serve as effective alkylating agents, such as building blocks for derivatives of fatty amines. 1-Bromo-3-chloropropane and phenylethylbromide are produced by related free radical additions to allyl chloride and styrene, respectively.
Dichloromethane and hydrogen bromide react to form bromochloromethane and dibromomethane, respectively:
HBr + CH2Cl2 → HCl + CH2BrCl
HBr + CH2BrCl → HCl + CH2Br2
These metathesis reactions illustrate the consumption of the stronger acid (HBr) and release of the weaker acid (HCl).
Allyl bromide is prepared by treating allyl alcohol with HBr:
CH2=CHCH2OH + HBr → CH2=CHCH2Br + H2O
HBr adds to alkynes to yield bromoalkenes. The stereochemistry of this type of addition is usually anti:
RC≡CH + HBr → RC(Br)=CH2
Also, HBr adds epoxides and lactones, resulting in ring-opening.
With triphenylphosphine, HBr gives triphenylphosphonium bromide, a solid “source” of HBr.
P(C6H5)3 + HBr → [HP(C6H5)3]+Br−
Inorganic chemistry
Vanadium(III) bromide and molybdenum(IV) bromide were prepared by treatment of the higher chlorides with HBr. These reactions proceed via redox reactions:
2 VCl4 + 8 HBr → 2 VBr3 + 8 HCl + Br2
Industrial preparation
Hydrogen bromide (along with hydrobromic acid) is produced by combining hydrogen and bromine at temperatures between 200 and 400 °C. The reaction is typically catalyzed by platinum or asbestos.
Laboratory synthesis
HBr can be prepared by distillation of a solution of sodium bromide or potassium bromide with phosphoric acid or sulfuric acid:
KBr + H2SO4 → KHSO4 + HBr
Concentrated sulfuric acid is less effective because it oxidizes HBr to bromine:
2 HBr + H2SO4 → Br2 + SO2 + 2 H2O
The acid may be prepared by:
reaction of bromine with water and sulfur:
2 Br2 + S + 2 H2O → 4 HBr + SO2
The above methods for producing hydrogen bromide can result in Br2 contamination, which can be eliminated by passing the gas through copper turnings or copper gauze at high temperatures, or by passing it through a phenol solution in tetrachloromethane or another suitable solvent at room temperature (producing 2,4,6-tribromophenol and more HBr in the process).
Safety
HBr is highly corrosive and irritating to inhalation.