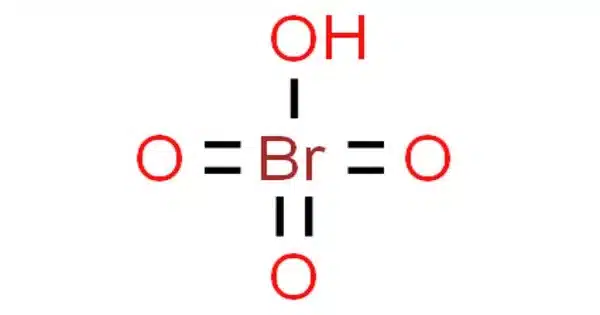
Perbromic acid is an inorganic chemical having the formula HBrO4. Perbromic acid is a white liquid with no discernible odor. It’s a bromine oxoacid with an oxidation state of 7+. It is not as common as some other halogen acids, and it is frequently manufactured for specialized chemical processes.
Perbromic acid is a powerful acid that is strongly oxidizing, though dilute perbromic acid solutions are sluggish oxidizing agents. It is the most unstable of the halogen(VII) oxoacids. On standing, it quickly decomposes to bromic acid and oxygen, releasing poisonous brown bromine fumes. It can be employed in the manufacture of perbromate salts by interacting with a base.
Properties
Perbromic acid is an unstable compound that cannot be created by displacing chlorine from perchloric acid, as periodic acid is; it can only be created by protonation of the perbromate ion. Perbromic acid is stable in aqueous solutions with a concentration of no more than 6M. Perbromic acid solutions larger than 6M are unstable in air, where the molecule decomposes autocatalytically.
- Chemical formula: BrHO4
- Molar mass: 144.908
- Melting point: decomposes before melting, unstable as solid
- Conjugate base: Perbromate
Perbromic acid is typically not stable in its pure form and is usually handled as a solution in water or other solvents. It can be prepared by mixing bromine pentafluoride (BrF5) with water, resulting in the formation of perbromic acid and hydrofluoric acid (HF) as byproducts.
Discovery
Perbromic acid was identified by decomposing a radioactive selenate sample, SeO4-, in which bromine crystals were subjected to gamma radiation.
Application
Perbromic acid is a powerful oxidizing agent that can cause intense reactions with reducing agents and organic compounds. Due to its instability and the availability of alternative, more frequent halogen acids such as hydrobromic acid (HBr) or bromine water for various chemical processes, it has limited practical applicability.
Safety
Because perbromic acid is corrosive and irritating, it is particularly hazardous to the skin, eyes, airways, and digestive tract. Excessive exposure can cause suffocation of the lungs, loss of consciousness, and death. Because of its toxicity, prolonged or repeated exposure may cause organ damage through the lungs, kidneys, and intestines.
Perbromic acid should be handled with carefully by trained specialists due to its reactivity and potential risks, and necessary safety precautions should be used when dealing with it in a laboratory setting.