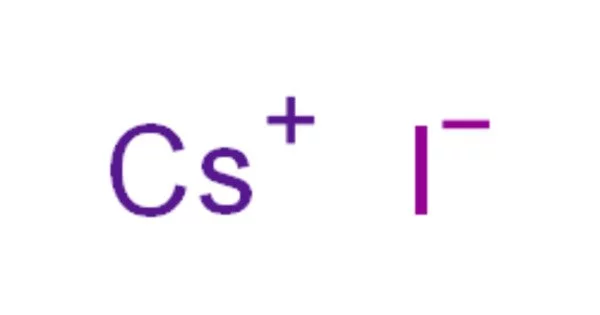Caesium iodide, also known as cesium iodide, is an ionic compound of caesium and iodine. It is hygroscopic and easily dissolves in water. Because of this property, it must be kept away from water and is difficult to polish. It is frequently used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are extremely efficient at extreme ultraviolet wavelengths.
Caesium Iodide is used to make cell windows and prisms for infrared applications up to 50 µm. It is also used in beam splitters for Fourier Transform Infrared (FTIR) devices. And in scintillators, where it may or may not be doped with Thallium.
Properties
Cesium iodide is a simple ionic salt. It has the capacity to undergo second order transformation from cubic B2 structure to body-centered tetragonal structure at low pressures. It is mostly used as input screens of X-ray image intensifiers.
- Chemical formula: CsI
- Molar mass: 259.809 g/mol
- Appearance: white crystalline solid
- Density: 4.51 g/cm3
- Melting point: 632 °C (1,170 °F; 905 K)
- Boiling point: 1,280 °C (2,340 °F; 1,550 K)
- Solubility in water: 848 g/L (25 °C)

Structure
The cubic CsCl crystal structure is found in bulk caesium iodide crystals, but the structure of nanometer-thin CsI films depends on the substrate material, which is CsCl for mica and NaCl for LiF, NaBr, and NaCl substrates.
Atomic chains of caesium iodide can be grown inside double-walled carbon nanotubes. Despite their lower mass, I atoms in such chains appear brighter than Cs atoms in electron micrographs. The charge difference between Cs atoms (positive), inner nanotube walls (negative), and I atoms explained this difference (negative). As a result, Cs atoms are drawn to the walls of the nanotube and vibrate more strongly than I atoms, which are pushed toward the nanotube axis.
Applications
Electromagnetic calorimetry is an important application of scintillator caesium iodide crystals in experimental particle physics. Pure CsI is a fast and dense scintillating material with a low light yield that rises dramatically with cooling. It exhibits two major emission components, one in the near ultraviolet region at 310 nm and the other at 460 nm. CsI has a high temperature gradient and is slightly hygroscopic.
Caesium iodide is used in Fourier transform infrared (FTIR) spectrometers as a beamsplitter. It has a greater transmission range than potassium bromide beamsplitters and can work into the far infrared. Optical-quality CsI crystals, on the other hand, are very soft and difficult to cleave or polish. They should also be coated (typically with germanium) and stored in a desiccator, to minimize interaction with atmospheric water vapors.
In addition to image intensifier input phosphors, caesium iodide is often also used in medicine as the scintillating material in flat panel x-ray detectors.