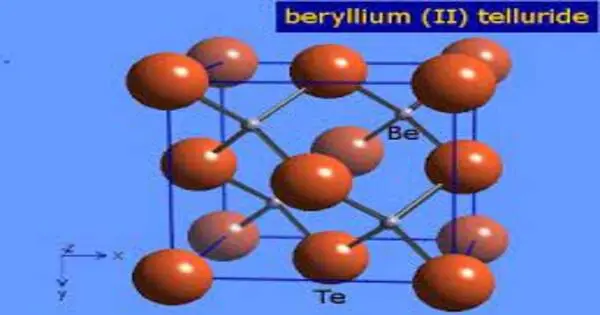
Beryllium telluride (BeTe) is a beryllium and tellurium chemical compound. It has a lattice constant of 0.5615 nm and is a crystalline solid. It is a semiconductor with an energy gap of approximately 3 eV. Toxicity has not been determined. When exposed to water, toxic hydrogen telluride gas is produced.
It functions as a semiconductor. Beryllium is an atomic number 4 lightweight alkaline earth metal. It is a relatively uncommon element that occurs naturally only in combination with other elements in minerals.
Properties
Beryllium telluride (BeTe) is a chemical compound whose molecular weight is 136.6122 g/mol. Its CAS number is 12232-27-8 and it has a density of 5.12 g/ cm3. Its structure is one of the sphalerite cF8 group with a space group of F43m. It is a crystalline solid with face-centered symmetry, a = 5.615 ? at 300 K.
- Chemical formula: BeTe
- Molar mass: 136.612 g/mol
- Density: 5.1 g/cm3
- Crystal structure: sphalerite, cF8, Space group = F-43m, No. 216
- Molecular Weight: 136.612182
- Class: telluride

Preparation
It can be prepared by heating the elements together in an inert atmosphere:
Be+ Te+ heat → BeTe
Be melts at 1287°C and Te melts at 450°C. A temperature of 750°C is sufficient to complete the reaction. BeTe has a lattice constant of 0.5615 nm and is a crystalline solid. It is a semiconductor with an energy gap of approximately 3 eV. Although the toxicity is unknown, both beryllium and tellurium are toxic. When exposed to water, toxic H2Te gas is produced.
Beryllium is a toxic bivalent element that is steel gray in color, strong, and light in weight, and is primarily used as a hardening agent in alloys. Beryllium has one of the light metals’ highest melting points. It has excellent thermal conductivity, is nonmagnetic, resists attack by concentrated nitric acid, and resists oxidation when exposed to air at standard temperatures and pressures.
Health effect
Beryllium is not a necessary element for humans; in fact, it is one of the most toxic chemicals known to man. It is a metal that can be extremely dangerous when inhaled by humans because it can damage the lungs and cause pneumonia.
The most well-known side effect of beryllium is berylliosis, a dangerous and long-lasting lung disorder that can also harm other organs such as the heart. This disease kills about 20% of all patients. Berylliosis is caused by inhaling beryllium in the workplace. People with weakened immune systems are especially vulnerable to this disease.