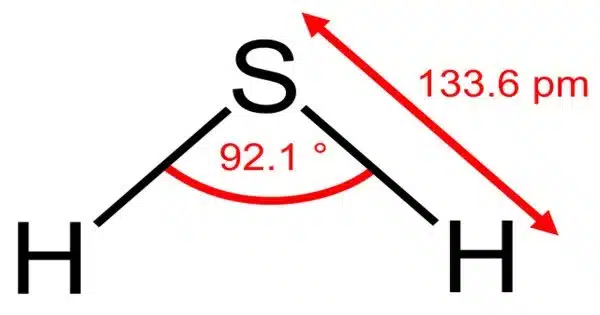
Hydrogen Sulfide is a chemical compound with the formula H 2S. It is a colourless chalcogen-hydride gas that is poisonous, corrosive, and flammable, with trace amounts in the ambient atmosphere emitting a characteristic rotten egg odour. stinkdamp is the underground mine gas term for foul-smelling hydrogen sulfide-rich gas mixtures. This gas is naturally occurring and can be found in a variety of environments, including swamps, volcanic areas, and the human body.
In 1777, Swedish chemist Carl Wilhelm Scheele is credited with discovering the chemical composition of purified hydrogen sulphide. The Royal Society of Chemistry and the International Union of Pure and Applied Chemistry no longer recommend the spelling hydrogen sulphide.
Properties
- Chemical formula: H2S
- Molar mass: 34.08 g·mol−1
- Appearance: Colorless gas
- Odor: Foul, pungent, like that of rotten eggs
- Density: 1.539 g.L−1 (0°C)
- Melting point: −85.5 °C (−121.9 °F; 187.7 K)
- Boiling point: −59.55 °C (−75.19 °F; 213.60 K)
- Solubility in water: 3.980 g dm−3 (at 20 °C)
- Vapor pressure: 1740 kPa (at 21 °C)
- Acidity (pKa): 7.0
- Conjugate acid: Sulfonium
- Conjugate base: Bisulfide
Production
Natural and industrial processes can both produce hydrogen sulphide. Volcanic activity, decay of organic matter in swamps, and certain biological processes are examples of natural sources. It is also produced by some bacteria during organic matter decomposition, which is why it is often associated with the odor of rotten eggs.
The most common way to obtain hydrogen sulphide is to separate it from sour gas, which is natural gas with a high H2S content. It can also be made by heating hydrogen with molten elemental sulphur to about 450 degrees Celsius. Hydrocarbons can be used as a hydrogen source in this process.
Sulfate-reducing (resp. sulfur-reducing) bacteria generate usable energy under low-oxygen conditions by using sulfates (resp. elemental sulfur) to oxidize organic compounds or hydrogen; this produces hydrogen sulfide as a waste product.
A standard lab preparation is to treat ferrous sulfide with a strong acid in a Kipp generator:
FeS + 2 HCl → FeCl2 + H2S
For use in qualitative inorganic analysis, thioacetamide is used to generate H2S:
CH3C(S)NH2 + H2O → CH3C(O)NH2 + H2S
Many metal and nonmetal sulfides, e.g. aluminium sulfide, phosphorus pentasulfide, silicon disulfide liberate hydrogen sulfide upon exposure to water:
6 H2O + Al2S3 → 3 H2S + 2 Al(OH)3
This gas is also produced by heating sulfur with solid organic compounds and by reducing sulfurated organic compounds with hydrogen.
Application
Hydrogen Sulfide is a precursor used in a variety of chemical synthesis processes. It is used to make sulphur and sulphur compounds, which are important in the production of chemicals and pharmaceuticals. In chemical reactions, it can be used as a reducing agent.
H2S is used to make metal sulphides, which are used in electronics, solar cells, and catalysts. It is used as a food preservative in the food industry to prevent microbial spoilage.
Safety
Hydrogen Sulfide is toxic to humans and most other animals because it inhibits cellular respiration in the same way that hydrogen cyanide does. When inhaled or ingested in large quantities, it causes rapid organ damage with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule.