Types of Galvanic Cell
In a typical electrochemical cell the cell potential is a direct consequence of the net chemical reaction. There may be another type of galvanic cell in which the chemical reaction is secondary, and the cell potential is a consequence of differences in concentration of either of the electrodes or of the electrolytic solution. These types of cells are called concentration cells. When the difference is in the concentration of electrodes, the cell is called electrode concentration cell. On the other hand if the potential arises due to the difference in the concentration of electrolyte, the cell is known as electrolytic concentration cell. There may be four different categories of galvanic cells.
(1) Electrochemical cell without transference
(2) Electrochemical cell with transference
(3) Concentration cell without transference
(4) Concentration cell with transference
- Type 1 cell
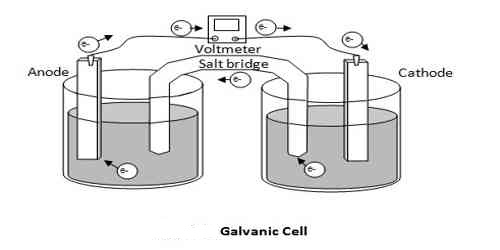
In this type of cell, the two electrodes are immersed in two electrolytic solutions separated by a salt bridge to avoid liquid junction potential. Daniel cell is an example of this Type of cell.
- Type 2 cell
In this case no salt bridge is used. There is a phase boundary in the form of a porous diaphragm and the ions move at different speeds through the liquid junction. This results in a junction potential, Ej. An example is:
Sn(s)│Sn2+ (aq)│Cu2+ (aq), Cu(s)
The cell potential will also include junction potential Ej
- Type 3 cell
As already mentioned, the concentration cell without transference may be of two types: (i) electrode concentration cells and (ii) electrolytic concentration cells.
Electrode concentration cells (without transference): This type of cell depends on the difference in concentrations at the electrodes. This may again be of two types: (a) Gas electrode: Two gas electrodes immersed in the same solution of the ions of the gaseous clement, but the gas bubbled around the electrodes have different pressures. Amalgam electrode: In this case the amount of metal mixed mercury is different.
Electrolytic concentration: cells (without transference): Two similar electrodes are immersed into two electrolytic solutions of different concentration.
- Type 4 cell
In this type of cells, the two electrodes are same but the concentrations of the electrolytes are different, and the two electrolytic solutions are placed in direct contact. There is no salt bridge between them.