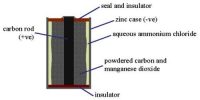
Normally the potential difference (voltage) measured across the electrodes is less than the maximum possible voltage of the cell. This is because the actual flow of electrons reduces the electrical pressure. Thus, a cell voltage has its maximum value when no current flows.
Electro-motive Force: Ecell
The maximum potential difference between the electrodes of a voltaic cell is referred to as the electro-motive force (also called emf) of the cell, or Ecell. It can be measured by an electronic digital voltmeter, which draws negligible current.
Potential Difference:Potential difference is a measure of the capacity of the cell to do work by moving electric charge through that potential difference. The amount of work that can be done is directly related to the enthalpy AH for the redox reaction (strictly AG, the free energy change which also incorporates entropy S).