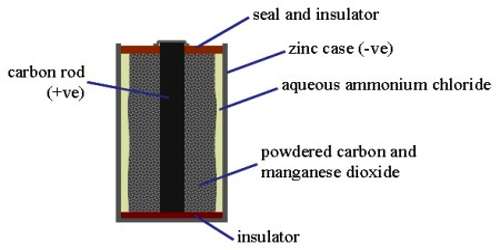
Leclanche’s dry cell is used as flash light battery and in the operation of transistorized equipments. In these cells a carbon rod acting as an inert electrode is surrounded by a paste consisting of manganese dioxide, graphite, a little zinc chloride and an excess of solid ammonium chloride placed in a zinc container. Zinc container acts as the other electrode. It uses a carbon rod as the cathode current collector with an electrolyte of ammonium chloride. The electrode reactions are-
Zn (s) ↔ Zn2+ (aq) + 2e–
2MnO2 (s) + 2NH4+ (aq) + 4H2O (l) + 2e– ↔ 2Mn (OH)3 (s) + 2NH4OH (aq)
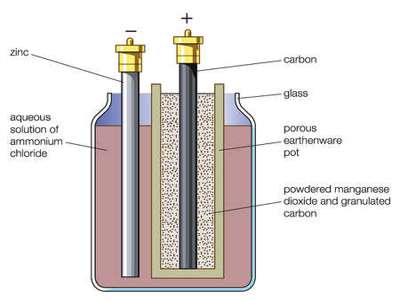
As the cell cannot be regenerated after the reactants have been used up it is composed of cheap material. The potential of the cell is about 1-5 V. Its variants have been in use for over a century. The performance of Leclanché cells improved by 700% between 1920 and 1990.
Types of Leclanche’s cell include:
(a) zinc (Carbon cathode)
(b) zinc chloride (Ammonium chloride electrolyte reinstated by zinc chloride)
(c) alkaline manganese (Ammonium chloride terminal displaced by potassium hydroxide)
Advantages
- Inexpensive materials
- Low cost
- Suitable for a wide range of consumer applications
- Interchangeable with alkaline batteries
Applications
General purpose, low cost applications; Remote controls, Flashlights, Clocks etc.