Fuel Cell
At present fossil fuel is the primary source of energy to meet our requirements. But the conversion of fossil fuel either to electrical energy or to thermal energy is an inefficient process because a significant portion of the energy is lost to the surrounding in the form of heat. Also, combustion of fossil fuel generates CO2 gas which is a greenhouse gas. Efforts are constantly being made to increase the efficiency and reduce the cost of conversion of fossil fuel by the electrochemical method in a device known as ‘fuel cell’.
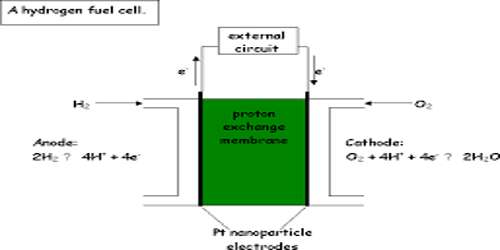
A device is also a form of galvanic cell that requires a continuous supply of reactants to keep it functioning. The simplest form of the fuel cell is a H2 – O2 fuel cell. In this form of fuel cell, hydrogen and oxygen gases are continuously bubbled through an electrolytic solution containing two inert electrodes. Usually, KOH solution is used as the electrolyte. The anode is made up of porous carbon-containing Ni, while the cathode is made up of porous carbon-containing Ni and NiO. A schematic diagram is shown in Figure.
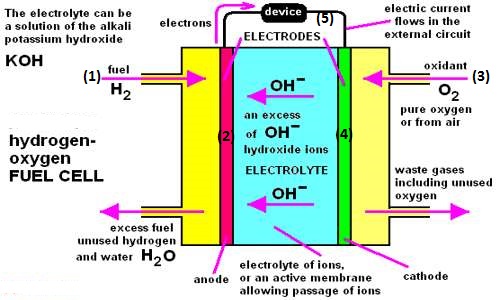
a stream of H2O, 2. porous cathode, 3. stream of O2, 4. porous anode 5. Electron flow through the external circuit
Figure: A H2 – O2 fuel cell
The reactions occurring at the electrodes are as follows-
Anode: 2H2 (g) + 4OH– (aq) → 4 H2O (l) + 4e–
Cathode: O2 (g) + 2 H2O (l) + 4e– → 4OH– (aq)
……………………………………………………….
Overall: 2H2 (g) + O2 (g) → 2 H2O (l)
The standard e.m.f. of this fuel cell has been calculated as +1.23 V. Thus the reaction is spontaneous under standard conditions. American Gemini space probes and Apollo moon probes used the H2 – O2 fuel cell for the first time. The astronauts used the product of the reaction as drinking water.
Other forms of fuel cells base been devised propane-oxygen (C3H8 – O2) Fuel Cell is one of them.