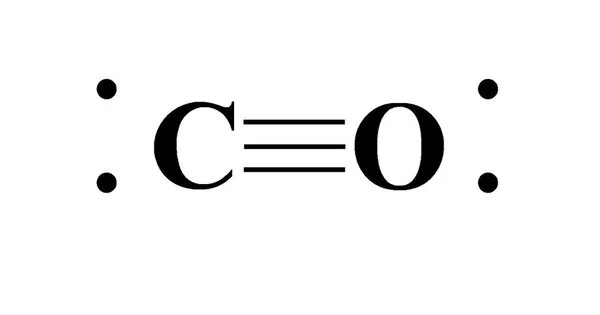
Carbon monoxide (CO) is a colorless, highly poisonous, odorless, tasteless, flammable gas with a density slightly less than that of air. Carbon monoxide is made up of one carbon atom and one oxygen atom linked together by a triple bond. It is the most basic molecule in the oxocarbon family. Carbonyl is the name given to the carbon monoxide ligand in coordination complexes. It is an important component in many industrial chemistry processes.
The most common source of carbon monoxide is partial combustion of carbon-containing compounds, which occurs when there is insufficient oxygen or heat to produce carbon dioxide. There are also numerous biological and environmental sources that generate and emit significant amounts of carbon monoxide. It is essential in the production of a wide range of compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change.
Carbon monoxide plays critical biological roles in all phylogenetic kingdoms. Many organisms, including humans, produce it. Carbon monoxide is a classic example of hormesis in mammalian physiology, where low concentrations act as an endogenous neurotransmitter (gasotransmitter) and high concentrations are toxic, resulting in carbon monoxide poisoning. It has isoelectronic properties with the cyanide anion CN–.
Physical and chemical properties
- Chemical formula: CO
- Molar mass: 28.010 g/mol
- Appearance: Colorless gas
- Odor: Odorless
- Density: 789 kg/m3, liquid; 1.250 kg/m3 at 0 °C, 1 atm; 1.145 kg/m3 at 25 °C, 1 atm
- Melting point: −205.02 °C (−337.04 °F; 68.13 K)
- Boiling point: −191.5 °C (−312.7 °F; 81.6 K)
- Solubility in water: 27.6 mg/L (25 °C)
- Solubility: soluble in chloroform, acetic acid, ethyl acetate, ethanol, ammonium hydroxide, benzene
Carbon monoxide is the simplest oxocarbon and is isoelectronic with other triply-bonded diatomic species possessing 10 valence electrons, including the cyanide anion, the nitrosonium cation, boron monofluoride and molecular nitrogen. It has a molar mass of 28.0, which, according to the ideal gas law, makes it slightly less dense than air, whose average molar mass is 28.8.
Carbon and oxygen are linked by a triple bond composed of two pi bonds and one sigma bond. The length of the bond between the carbon and oxygen atoms is 112.8 pm. This bond length is consistent with a triple bond, similar to that of molecular nitrogen (N2), which has a similar bond length (109.76 pm) and nearly the same molecular mass. Carbon-oxygen double bonds are significantly longer, for example, 120.8 pm in formaldehyde. The boiling (82 K) and melting (68 K) points are very close to those of N2 (77 K and 63 K, respectively). The bond-dissociation energy is 1072 kJ/mol, which is greater than the bond-dissociation energy of N2 (942 kJ/mol) and represents the strongest chemical bond known.
The ground electronic state of carbon monoxide is a singlet state since there are no unpaired electrons.
Production
Thermal combustion is the most common source of carbon monoxide. Carbon monoxide is produced by the partial oxidation of carbon-containing compounds; it forms when there is insufficient oxygen to produce carbon dioxide (CO2), such as when using a stove or an internal combustion engine in an enclosed space. During World War II, for example, a gas mixture containing carbon monoxide was used to keep motor vehicles running in parts of the world where gasoline and diesel fuel were scarce. With a few exceptions, external charcoals or wood gas generators were installed, and the mixture of atmospheric nitrogen, hydrogen, carbon monoxide, and small amounts of other gases produced by gasification was piped to a gas mixer.
During the oxidative processes used to produce chemicals, a large amount of CO byproduct is produced. As a result, the process off-gases must be purified. On the other hand, significant research efforts are expended in order to optimize process conditions, develop catalysts with improved selectivity, and comprehend the reaction pathways leading to the target product and side products.
Occurrence
Carbon monoxide can be found in a variety of natural and man-made environments. Photochemical degradation of plant matter, for example, is estimated to produce 60 billion kilograms per year.