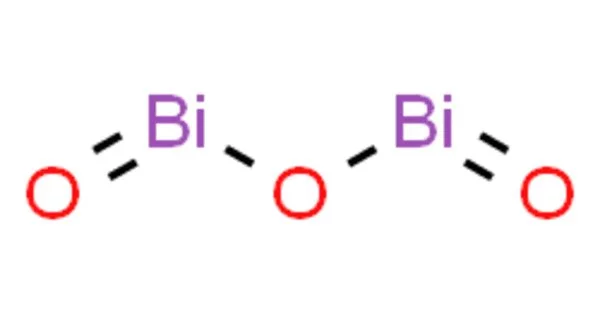
Bismuth(III) oxide is the most important industrially important bismuth compound. It’s also a common place to start in bismuth chemistry. It occurs naturally as the minerals bismite (monoclinic) and sphaerobismoite (tetragonal, much rarer), but it is most commonly obtained as a byproduct of the smelting of copper and lead ores. As a substitute for red lead, dibismuth trioxide is commonly used in fireworks to produce the “Dragon’s eggs” effect.
Bismuth trioxide is found naturally as the mineral bismite. The oxide is used to fireproof papers and polymers, to enamel cast iron ceramics, and to make disinfectants.
Properties
Bismuth(III) oxide is formed by heating the metal or its carbonate in air. It is unmistakably a basic oxide, dissolving readily in acid solutions and, unlike arsenic or antimony compounds, not amphiprotic in solution, though it forms stoichiometric addition compounds when heated with oxides of several other metals.
- Chemical formula: Bi2O3
- Molar mass: 465.958 g·mol−1
- Appearance: yellow crystals or powder
- Odor: odorless
- Density: 8.90 g/cm3, solid
- Melting point: 817 °C (1,503 °F; 1,090 K)
- Boiling point: 1,890 °C (3,430 °F; 2,160 K)
- Solubility in water: insoluble
- Solubility: soluble in acids
- Crystal structure: monoclinic, mP20,
- Coordination geometry: pseudo-octahedral

Structure
The structures adopted by Bi2O3 differ significantly from those of arsenic(III) oxide, As2O3, and antimony(III) oxide, Sb2O3.
Bismuth oxide, Bi2O3 has five crystallographic polymorphs. The room temperature phase, α-Bi2O3 has a monoclinic crystal structure. There are three high-temperature phases, a tetragonal β-phase, a body-centred cubic γ-phase, a cubic δ-Bi2O3 phase, and an ε-phase. The room temperature α-phase has a complex structure with layers of oxygen atoms with layers of bismuth atoms between them. The bismuth atoms are in two different environments which can be described as distorted 6 and 5 coordinates respectively.
Preparation
The trioxide can be prepared by ignition of bismuth hydroxide. Bismuth trioxide can be also obtained by heating bismuth subcarbonate at approximately 400 °C.
Reactions
When atmospheric carbon dioxide (CO2) is dissolved in water, it easily reacts with Bi2O3 to form bismuth subcarbonate. Bismuth oxide is classified as a basic oxide, which accounts for its high reactivity with CO2. However, when acidic cations such as Si(IV) are introduced into the bismuth oxide structure, the reaction with CO2 does not occur.
Bismuth(III) oxide reacts with either concentrated aqueous sodium hydroxide and bromine or concentrated aqueous potassium hydroxide and bromine to form sodium bismuthate or potassium bismuthate.
Uses
Bismuth(III) oxide is used in the preparation of BiFeO3perovskite nanoparticles. It is used in disinfectants, magnets, glass, rubber, vulcanization, fireproofing papers, polymers, and catalysts. As a replacement for red lead, bismuth trioxide produces the “dragon’s eggs” effect in fireworks. Because of their low cost and ease of use, bismuth(III) compounds are appealing reagents and catalysts in organic synthesis. Bismuth trioxide nanoparticles are also important in high-energy gas generators.
Medical device usage
Bismuth oxide is used in dental materials on occasion to make them more X-ray opaque than the surrounding tooth structure. Bismuth (III) oxide, in particular, has been used in hydraulic silicate cements (HSC), initially in “MTA” (trade name for “mineral trioxide aggregate”) from 10 to 20% by mass with a mixture of primarily di- and tricalcium silicate powders.
Some resin-based materials also contain an HSC containing bismuth oxide. Bismuth oxide has allegedly caused problems because it is claimed not to be inert at high pH, specifically that it slows the setting of the HSC, but also that it can lose color over time due to exposure to light or reaction with other materials used in the tooth treatment, such as sodium hypochlorite.