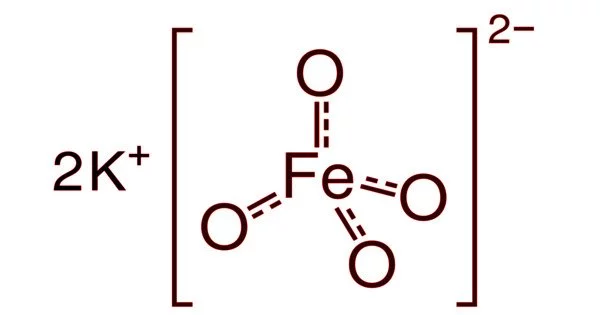
The chemical compound potassium ferrate has the formula K2FeO4. This paramagnetic purple salt is a rare example of an iron(VI) compound. Iron has the oxidation state +2 or +3 (Fe2+ or Fe3+) in the majority of its compounds. It is a strong oxidizing agent due to its high oxidation state.
Because the byproducts of its use, iron oxides, are environmentally benign, it has attracted interest for applications in “Green Chemistry.” In contrast, some related oxidants, such as chromate, are considered hazardous to the environment. The main issue with using K2FeO4 is that it is frequently overly reactive.
Properties
- Chemical formula: K2FeO4
- Molar mass: 198.0392 g/mol
- Appearance: Dark purple solid
- Density: 2.829 g/cm3, solid
- Melting point: >198 °C (decomposition temp)
- Solubility in water: soluble in 1M KOH
- Solubility in other solvents: reacts with most solvents
- Crystal structure: K2SO4 motif
Synthesis and structure
Georg Ernst Stahl (1660–1734) was the first to discover that igniting a mixture of potassium nitrate (saltpetre) and iron powder dissolved in water resulted in a purple solution. Later, Edmond Frémy (1814–1894) discovered that the fusion of potassium hydroxide and iron(III) oxide in air produced a soluble compound:
4 KOH + Fe2O3 + 3 O2 → 2 K2FeO4 + 2 H2O
The composition corresponded to that of potassium manganate. In the laboratory, K2FeO4 is prepared by oxidizing an alkaline solution of an iron(III) salt with concentrated chlorine bleach.:
3 ClO– + 3 Fe(OH)3(H2O)3 + 4 K+ + 4 OH– → 3 Cl– + 2 K2FeO4 + 11 H2O
The salt is isostructural with K2MnO4, K2SO4, and K2CrO4. The solid consists of K+ and the tetrahedral FeO42- anion, with Fe-O distances of 1.66 Å. The poorly soluble barium salt, BaFeO4, is also known.

Decomposes
The main difficulty with the use of K2FeO4 is that it is often too reactive, as indicated by the fact that it decomposes in contact with water, especially in acidic water:
4 K2FeO4 + 4 H2O → 3 O2 + 2 Fe2O3 + 8 KOH
At high pH, aqueous solutions are stable. The deep purple solutions are similar in appearance to potassium permanganate (KMnO4). It is stronger oxidizing agent than the latter. As a dry solid, K2FeO4 is stable.
It decomposes with the evolution of O2 in neutral water, particularly quickly in acidic water. The deep purple solutions resemble potassium permanganate in appearance. K2FeO4 is a more powerful oxidizing agent than KMnO4.
K2FeO4 has been dubbed a “green oxidant” because the byproducts of its redox reactions are rust-like iron oxides. It has been used in waste-water treatment as an organic contaminant oxidant and as a biocide. The resulting reaction product is iron(III) oxyhydroxide, which is an excellent flocculant. K2FeO4 oxidizes primary alcohols in organic synthesis. In contrast, related oxidants such as chromate are considered hazardous to the environment. K2FeO4 has also sparked interest as a possible cathode material in a “super iron battery.”
Stabilized forms of potassium ferrate have been proposed for the removal of transuranic species, both dissolved and suspended, from aqueous solutions. Tonnage amounts were proposed to aid in the cleanup of the Chernobyl disaster in Belarus. This new technique was successfully used to remove a wide range of heavy metals.
Applications
From 1987 to 1992, work was done in the Laboratories of IC Technologies Inc. in collaboration with ADC Laboratories on the use of potassium ferrate precipitation of transuranics and heavy metals. Transuranic species were removed from samples from various Department of Energy nuclear sites in the United States. It has been proposed as a bleeding stopper for fresh wounds.